Abstract
Background
Echocardiographic longitudinal markers of right ventricular (RV) systolic function are commonly depressed after coronary artery bypass graft surgery (CABG) despite an uncomplicated course and good clinical recovery. The exact timing and cause of these changes is unknown. The aim of this observational study was to monitor echocardiographic markers of RV systolic function intraoperatively during CABG. We used angle-independent speckle tracking to measure the primary endpoints of tricuspid annular plane systolic excursion (TAPSE) and tricuspid annular systolic velocity (S′) before and after pericardiotomy.
Methods
Twenty-four patients undergoing elective on-pump CABG were enrolled in the study. Speckle tracking-derived TAPSE, S’, free wall systolic strain, RV outflow tract strain, colour tissue Doppler-derived isovolumic acceleration (IVA) and two-dimensional RV dimensions and fractional area change (FAC) were measured at three intraoperative time points: 1) after sternotomy immediately prior to pericardiotomy; 2) after pericardiotomy and placement of pericardial retraction sutures; and 3) following cardiopulmonary bypass after chest closure.
Results
Adequate image quality to perform speckle tracking measurements was obtained in twenty-one patients. We found that there were no significant changes to echocardiographic parameters of RV systolic function between pre- and post-pericardiotomy. The mean (SD) of the primary endpoints were: TAPSE [28.1 (5.1) mm vs 27.7 (7.4) mm, respectively; mean difference, −0.4 mm; 97.5% confidence interval (CI), −4.0 to 3.1; P = 0.76] and S′ [10.4 (2.1) cm·sec−1 vs 10.8 (1.9) cm·sec−1, respectively; mean difference, 0.4 cm·sec−1; 97.5% CI, −0.9 to 1.7; P = 0.48]. Significant reductions in the parameters of RV systolic function were found only after cardiopulmonary bypass and chest closure.
Conclusion
Pericardial opening and suspension had no significant effect on the indices of RV systolic function derived from speckle tracking or colour tissue Doppler.
Résumé
Contexte
Les marqueurs échocardiographiques longitudinaux de la fonction systolique ventriculaire droite (VD) sont habituellement déprimés après une chirurgie de pontages aorto-coronariens (PAC) malgré une évolution non compliquée et une bonne récupération clinique. Le moment exact et la cause de ces modifications sont inconnus. L’objectif de cette étude observationnelle était de surveiller l’évolution des marqueurs échocardiographiques de la fonction systolique du VD au cours d’une chirurgie de PAC. Nous avons utilisé un suivi du « speckle » indépendant de l’angle pour mesurer les critères d’évaluation principaux qui étaient le déplacement systolique du plan de l’anneau tricuspidien (TAPSE) et la vélocité systolique de l’anneau tricuspidien (S’) avant et après la péricardiotomie.
Méthodes
Vingt-quatre patients subissant une chirurgie de PAC planifié sous circulation extracorporelle ont été inclus dans l’étude. Les index échocardiographiques de la fonction du VD (TAPSE, S’, tension systolique de la paroi libre, tension de la chambre d’éjection du VD, accélération tissulaire isovolumique mesurée par Doppler, dimensions bidimensionnelles [2D] du VD et changement de la fraction d’éjection) ont été mesurés à trois moments peropératoires différents: 1) après la sternotomie, immédiatement avant la péricardiotomie; 2) après la péricardiotomie et la mise en place des sutures de rétraction péricardique; et 3) après le pontage cardiopulmonaire et la fermeture du thorax.
Résultats
Une qualité d’image adéquate pour effectuer les mesures de suivi de « speckle » a été obtenue chez vingt et un patients. Après la péricardiotomie, le TAPSE moyen (ÉT) et le S’ (ÉT) ne différaient pas d’avant la péricardiotomie: TAPSE (27,7 [7,4] mm contre, respectivement, 28,1 [5,1] mm; différence moyenne, -0,4 mm; intervalle de confiance à 97,5 % [IC]: -4,0 à -3,1; P = 0,76) et S’ (10,8 [1,9] cm·s-1, respectivement, 10,4 [2,1] cm·s-1; différence moyenne, 0,4 cm·s-1; IC à 97,5 %: -0,9 à 1,7; P = 0,48). De plus, aucun autre index de la fonction ventriculaire droite n’a été différent après la péricardiotomie. Des baisses significatives des paramètres de la fonction systolique du VD n’ont été trouvées qu’après la pompe cardiopulmonaire et fermeture du thorax.
Conclusion
L’ouverture et la suspension du péricarde n’ont pas eu d’effets significatifs sur les index de la fonction systolique du VD dérivés du suivi de speckle ou du Doppler couleur tissulaire.
Similar content being viewed by others
It has previously been shown that echocardiographic longitudinal markers of right ventricular (RV) systolic function, such as tricuspid annular plane systolic excursion (TAPSE) and tricuspid annular systolic velocity (S′), are depressed after elective coronary artery bypass graft surgery (CABG), whether on or off cardiopulmonary bypass (CPB).1,2,3,4,5,6 Similar changes have also been noted after mitral valve repair7 and aortic valve replacement.8,9,10 Furthermore, depressed longitudinal function may persist for one year or more despite a successful and otherwise uncomplicated clinical course.3,5,11,12,13 Proposed mechanisms for this observation include the loss of pericardial support consequent to the necessary pericardiotomy for cardiac surgery, ischemic injury to the right ventricle (RV) due to incomplete myocardial protection, or formation of adhesions between the RV and the surrounding tissues, all of which could then alter RV geometry and contraction patterns.
Previous intraoperative studies using transesophageal echocardiography (TEE) have found a reduction in S′ velocity immediately after pericardial opening,1,9 consistent with what might be expected from geometric changes in the RV. However, as the TEE midesophageal four-chamber view does not allow for accurate Doppler alignment with the direction of motion of the lateral tricuspid annulus, errors in S′ measurements can occur.14 Furthermore, intraoperative studies have not examined angle-independent or less load-dependent indices of RV systolic function such as strain derived from speckle tracking and isovolumic acceleration (IVA), respectively.
Speckle tracking is a technology that uses specialized software to track B-mode tissue markers in order to measure myocardial displacement, velocity, and strain. Unlike tissue Doppler, measurements are angle independent and thus unaffected by any difference between the direction of the ultrasound beam and the direction of movement of the tricuspid annulus and RV myocardial free wall. This may allow for more accurate measurements of annular displacement and velocity as well as myocardial deformation when using TEE. In addition to speckle tracking, the RV IVA is a relatively load-independent measure of contractility that can be measured using colour tissue Doppler imaging.15,16 This measurement may be less affected by any changes in hemodynamic loading at the time of pericardial opening.
In this study, we aimed to assess RV systolic function more accurately using speckle tracking and colour tissue Doppler and to determine whether pericardial opening is responsible for the postoperative changes observed in RV function. We hypothesized that the loss of pericardial support is not a significant contributor to the decrease in RV longitudinal function in the perioperative period. The primary endpoints measured were TAPSE and S′ derived from speckle tracking before and after pericardiotomy.
Methods
The St. Michael’s Hospital Research Ethics Board approved the protocol (September 2015). Eligible patients were approached preoperatively and written informed consent was obtained prior to conducting any procedures related to the study. Twenty-four patients undergoing elective CABG at St. Michael’s Hospital, Toronto were recruited from September 2015 to December 2015. Patients were excluded if they had a contraindication to TEE, left ventricular systolic dysfunction (defined as a left ventricular ejection fraction [LVEF] < 40% on preoperative transthoracic echocardiography), RV systolic dysfunction (defined as TAPSE < 17 mm on preoperative transthoracic echocardiography), previous cardiac surgery, hemodynamically significant valvular dysfunction (moderate or more stenosis/regurgitation of the tricuspid, pulmonary, mitral, or aortic valve), atrial fibrillation or flutter, or if they were < 18 yr of age, pregnant, or did not speak English.
In accordance with normal clinical practice, electrocardiogram monitoring was used for all patients, and a radial arterial cannula and pulmonary artery catheter (7.5 Fr, Edwards Lifesciences, Irvine, CA, USA) were placed. General endotracheal anesthesia was induced with midazolam, sufentanil, and rocuronium and maintained with sevoflurane. A multiplane TEE probe (GE Vivid E9 System, Milwaukee, WI, USA) was inserted. Three measurement time points were defined: 1) after sternotomy, immediately before pericardial opening; 2) after pericardial opening and placement of pericardial retraction sutures; and 3) following CPB after chest closure. At each of these three time points, mechanical ventilation was suspended for both hemodynamic and echocardiographic measurements. No intravenous fluid boluses, vasopressors, or inotropes were administered between the first and second time points.
Hemodynamic measurements
At each time point, image acquisition was performed simultaneously with hemodynamic measurements over a period of approximately two to three minutes. Mean arterial blood pressure (MAP), mean pulmonary artery pressure (mPAP), central venous pressure (CVP), pulmonary capillary wedge pressure (PCWP), and heart rate (HR) were recorded. Cardiac output (CO) was measured by taking the average of three pulmonary artery catheter thermodilution measurements using 10 mL of room temperature saline, and stroke volume (SV) was calculated by dividing CO by HR.
Echocardiographic measurements
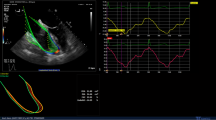
The midesophageal four-chamber view centred on the RV and the midesophageal RV inflow-outflow view were obtained. Imaging of eight consecutive beats was digitally recorded for later analysis. The sector width and depth were adjusted to achieve a frame rate of approximately 50-60 Hz. The transgastric RV inflow view was obtained and modified to align tricuspid annular motion with the Doppler plane at the edge of the two-dimensional (2D) sector by advancing the probe further into the stomach and adjusting the multiplane angle to approximately 130-160°. A colour tissue Doppler sector was applied to the tricuspid annular region where sector width and depth were adjusted to obtain a colour tissue Doppler frame rate of at least 200 Hz, as described previously14 (Fig. 1). Eight consecutive beats were digitally recorded for later analysis. The same observer used a GE workstation to analyze all echocardiographic images offline.
Right ventricular dimensions and fractional area change
From the images obtained in the midesophageal four-chamber RV view, standard measurements of RV basal diameter (RVD1) and RV mid-cavity diameter (RVD2) were obtained and expressed in millimetres. The RV fractional area change (RVFAC) was measured by tracing the endocardium in diastole and systole and expressed as a percentage. A measurement of the proximal right ventricular outflow tract diameter (RVOTprox) was taken in the midesophageal RV inflow-outflow view and expressed in millimetres. All measurements were averaged over three normally conducted beats.
Colour tissue Doppler IVA
In the modified transgastric RV inflow view, a 6 × 6 mm sample volume was applied to the tricuspid annulus in the colour tissue Doppler sector, as previously described.15 Using quantitative analysis software (GE, Milwaukee, WI, USA), the IVA was measured as the gradient of the annular velocity during the pre-ejection phase and expressed in m·sec−2. Measurements were averaged over five normally conducted beats (Fig. 1).
Speckle tracking analysis
All speckle tracking analysis was performed using GE EchoPAC quantitative analysis software (GE, Milwaukee, WI, USA). From the midesophageal four-chamber RV view, a region of interest that included the entire RV was used to calculate 2D systolic strain. As there was no dedicated RV longitudinal strain package available at the time of the study, the LV longitudinal strain package was used to analyze the RV, as described previously.17 The software automatically divides the RV into six segments, three in the free wall and three in the septum (Fig. 2). Only the free wall segments were used for analysis, otherwise known as free wall strain.18 Additionally, only beats with software-approved tracking quality in the three free wall segments were used for analysis. In the speckle tracking analysis for strain, RV free wall longitudinal peak systolic strain was recorded as the average of the three free wall segments (entire RV free wall) and expressed as a percentage (Fig. 3). Analysis for displacement and velocity at the lateral tricuspid annulus yielded TAPSE expressed in millimetres and S′ expressed in cm·sec−1, respectively. All measurements were averaged over three normally conducted beats with adequate tracking quality.
From the midesophageal RV inflow-outflow view, a region of interest was described from the tricuspid annulus to the pulmonary valve that was divided into six equal segments, as determined by the software package. The RVOT longitudinal peak systolic strain was recorded as the average of all six segments and was averaged over three normally conducted beats (Fig. 2).
Statistical analysis
Previous studies examining RV systolic function in the perioperative period have most commonly examined TAPSE and S′. For the purpose of sample size calculation, a 20% reduction in TAPSE and S′ at pericardiotomy was considered clinically significant. With a normal mean (standard deviation [SD]) TAPSE of 25 (5) mm, α = 0.025, β = 0.20, and a two-tailed analysis, a sample size of approximately 19 patients would be required. Similarly, with a normal mean (SD) S′ of 10 (2) cm·sec−1, α = 0.025, β = 0.20, and a two-tailed analysis, a sample size of approximately 19 patients would be required. We estimated that 80% of patients would have image quality adequate for speckle tracking analysis at all three time points and thus decided on a sample size of 24 patients.
The primary endpoint was the comparison of TAPSE and S′ before and after pericardiotomy. Secondary endpoints included comparisons of TAPSE and S′ data between pre-pericardiotomy and post-chest closure as well as all comparisons of RV free wall strain, RVOT strain, IVA, RVFAC, RVD1, RVD2 and RVOTprox. Hemodynamic data were reported to provide an understanding of the hemodynamic conditions at the times of measurement, but they were not considered outcome data.
Data were tested for normality using the D’Agostino-Pearson omnibus normality test. Unless otherwise stated, all data are expressed as mean (SD), with the 97.5% confidence interval (CI) reported as indicated. A paired two-tailed Student’s t test was used to test for difference in each of the echocardiographic and hemodynamic variables between pre-pericardiotomy and post-pericardiotomy and between pre-pericardiotomy and post chest closure. Pre-pericardiotomy was compared with post-pericardiotomy to test the study hypothesis and with post chest closure to test for the reductions in echocardiographic markers of RV systolic function observed in previous studies. After a Bonferroni correction based on comparing the two primary echocardiographic endpoints before and after pericardiotomy, statistical significance was assumed when P = 0.025. No additional multiple comparison adjustment was made for the exploratory secondary endpoints. Statistical analyses were performed using GraphPad Prism software version 7.0 (GraphPad Software, La Jolla, CA, USA).
Results
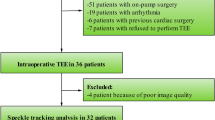
Twenty-four patients undergoing elective CABG were recruited. Three patients were excluded because echocardiographic image quality was not adequate for speckle tracking analysis at all three time points. There were 18 male and three female patients. Mean (SD) age, height, weight, and body surface area were: 70 (8) yr, 169 (7) cm, 84 (19) kg, and 2.0 (0.2) m2 respectively. The mean (SD) preoperative LVEF and CPB time were 62 (7)% and 78 (24) min, respectively. Mean (SD) cross-clamp time using antegrade cardioplegia was 67 (23) min. At the time of chest closure, a norepinephrine infusion with a median infusion rate of 0.04 µg·kg−1·min−1 was administered to all patients to maintain stable hemodynamic conditions. No patient was administered dobutamine, milrinone, epinephrine, or vasopressin at any time during the study. Two of the 21 patients were paced via an RV epicardial pacing lead at the time of chest closure. No patient was paced at the times of the pre-pericardiotomy or post-pericardiotomy measurements.
Hemodynamic measurements
Hemodynamic measurements are listed in Table 1. Between pre-pericardiotomy and post-pericardiotomy, there was a significant reduction in both CVP and MAP but no significant change in HR, mPAP, PCWP, SV, or CO. Between pre-pericardiotomy and chest closure, there was a significant increase in HR and a reduction in SV, but there were no changes in MAP, mPAP, CVP, PCWP, or CO.
Echocardiographic measurements
Echocardiographic measurements are shown in Table 2. There were no significant changes to echocardiographic parameters of RV systolic function between pre- and post-pericardiotomy. The mean (SD) of the primary endpoints were: TAPSE [28.1 (5.1) mm vs 27.7 (7.4) mm, respectively; mean difference, −0.4 mm; 97.5% CI, −4.0 to 3.1; P = 0.76] and S′ [10.4 (2.1) cm·sec−1 vs 10.8 (1.9) cm·sec−1, respectively; mean difference, 0.4 cm·sec−1; 97.5% CI, −0.9 to 1.7; P = 0.48]. Pericardial opening was not associated with any significant change in RV free wall strain, RVOT strain, IVA or RVFAC. Pericardial opening was only associated with an increase in the RVOTprox diameter.
When comparing pre-pericardiotomy to post chest closure values, there was a significant reduction in TAPSE, S′, RV free wall strain, and IVA (Table 2). There was no significant change in RVD1, RVD2, RVOTprox, RVFAC, or RVOT strain.
Discussion
In this study, we found that the opening and suspension of the pericardium did not result in any significant changes in echocardiographic parameters of RV systolic function. There were also no associated changes in RV dimensions, except for a small increase in the RVOTprox dimension. At the end of surgery, all echocardiographic markers of RV systolic function were significantly depressed, except for RVOT strain. There was no significant change in RV dimensions when compared with pre-pericardiotomy.
Longitudinal markers of RV function, such as TAPSE and S′, are well described to be depressed after cardiac surgery.1,2,3,4,5,6,7,8,9,10,11,12 A variety of hypotheses have been proposed to explain the reduction in TAPSE and S′ after uncomplicated cardiac surgery. Such theories include pericardial disruption, injury to the right atrium from venous cannulation, ischemic injury to the RV due to incomplete myocardial protection while on CPB, and formation of adhesions between the right ventricle and surrounding mediastinal tissues.8 Reductions in TAPSE and S′ after elective CABG may also result from conformational changes, such as altered geometry or contraction patterns, rather than a worsening of RV systolic function per se, as postoperative exercise capacity is increased,12 three-dimensional (3D) right ventricular ejection fraction (RVEF) by cardiac magnetic resonance imaging is unchanged,13 and CO is not reduced.13 Nevertheless, it remains unclear precisely when and why these indices of RV systolic function are reduced.
Unsworth et al. performed a study examining a group of nine patients using intraoperative pulse wave Doppler from the TEE midesophageal four-chamber view to measure peak S′ velocity continually throughout elective CABG.1 They found that the majority of the reduction in RV S′ occurred within minutes of opening the pericardium, with a 43% reduction three minutes after opening, a 54% reduction five minutes after opening, and a 61% reduction at the end of the operation. A second study performed by Unsworth et al. examined a group of 34 patients undergoing one of several procedures involving full pericardial opening (conventional on-pump CABG or aortic valve replacement [AVR]), limited pericardial opening (robotic CABG or minimally invasive AVR), or no pericardial opening (mediastinal mass excision).9 They found that a significant reduction in RV S′ velocities occurred only in patients undergoing full pericardial opening and occurred within five minutes of pericardial opening. They concluded that pericardial incision was the likely cause of reduced RV long-axis function after cardiac surgery. However, accurate measurement of velocity with Doppler is dependent on the alignment of the ultrasound beam with the direction of movement of the structure of interest. This particular alignment with the lateral tricuspid annulus is difficult to achieve in the midesophageal four-chamber view, which may lead to inaccurate or inconsistent velocity measurements.14
In a group of 30 patients undergoing elective surgical AVR randomized to either suture closure of pericardium or leaving the pericardium open, Lindquist et al. found that closing the pericardium made no difference to the typical postoperative reduction in TAPSE and RV S′.8 In a further study on pericardial closure, Lindstrom et al. examined a group of 17 patients undergoing CABG and performed closure of the pericardium with a biodegradable synthetic patch in six patients, while leaving the pericardium open in 11. On short- and long-term follow-up, they found a persistent postoperative reduction in tricuspid annular motion in both groups.19 Pinto et al. also found no change in tricuspid annular motion with opening the chest and pericardium in a group of patients undergoing cardiac surgery. They found that patients developed a reduction in tricuspid annular motion only after CPB.20
These investigations support our findings of preserved RV systolic function without significant changes in geometry after pericardiotomy, as measured by TAPSE, S′, and RVFAC. Additionally, the angle-independent measures of speckle tracking-derived strain in both the free wall and RVOT were also unaffected. The IVA, a relatively load-independent measure of RV systolic function was also unaffected. Our findings indicate that pericardiotomy did not result in large enough changes in geometry to influence the load-dependent measures. Although there was an increase in the RVOTprox dimension after pericardiotomy, there was no statistically significant change in strain (Table 2). Given the unchanged geometric dimensions at the end of surgery, the reductions in load-dependent and relatively load-independent measures of systolic function are the result of other processes inherent to cardiac surgery after CPB. Their reported persistence long into the postoperative period in the face of preserved exercise capacity is more difficult to explain. Preservation of RVOT strain might be an indicator of changes in contraction patterns in the postoperative period. There was no longer term follow-up for this parameter.
There were several limitations to this study. The RV is difficult to image in its entirety in one view and is difficult to model. A 3D RV function analysis, such as 3D RVEF and speckle tracking, might have been superior to 2D only. Subtle changes in other areas of the RV myocardium might have been detected. Only longitudinal strain was analyzed, whereas analysis of transmural or circumferential strain might have provided some additional clues into whether contraction patterns change in response to pericardiotomy or surgery itself. Additionally, patients did not undergo long-term follow-up with a transthoracic echocardiogram to assess whether any aspect of recovery was apparent at a later date or whether any changes in contraction patterns evolved in the face of a persistent depression of RV longitudinal strain. Hemodynamic conditions post-bypass were slightly different to pre-bypass, with a higher heart rate and lower stroke volume, which should be considered when comparing echocardiographic parameters at these two time points. Finally, multiple secondary endpoints were explored with no additional statistical adjustment made for these comparisons and consequently the significance of any differences should be interpreted with caution.
Conclusion
We found that TAPSE and S′, as measured by angle-independent speckle tracking, were unaffected by pericardial opening and suspension. Pericardiotomy had minimal effect on RV dimensions and no effect on RVFAC, RV free wall strain, RVOT strain, or IVA. All measures of RV systolic function, except RVOT strain, were depressed at the end of cardiac surgery. We conclude that factors other than the loss of pericardial support are responsible for the depression of longitudinal echocardiographic markers of RV systolic function after cardiac surgery.
References
Unsworth B, Casula RP, Kyriacou AA, et al. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J 2010; 159: 314-22.
Raina A, Vaidya A, Gertz ZM, Chambers S, Forfia PR. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: implications for post-surgical assessment of right ventricular function. J Heart Lung Transplant 2013; 32: 777-83.
Roshanali F, Yousefnia MA, Mandegar MH, Rayatzadeh H, Alinejad S. Decreased right ventricular function after coronary artery bypass grafting. Tex Heart Inst J 2008; 35: 250-5.
Michaux I, Filipovic M, Skarvan K, et al. A randomized comparison of right ventricular function after on-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2011; 141: 361-7.
Diller GP, Wasan BS, Kyriacou A, et al. Effect of coronary artery bypass surgery on myocardial function as assessed by tissue Doppler echocardiography. Eur J Cardiothorac Surg 2008; 34: 995-9.
Khani M, Hosseintash M, Foroughi M, Naderian M, Khaheshi I. Assessment of the effect of off-pump coronary artery bypass (OPCAB) surgery on right ventricle function using strain and strain rate imaging. Cardiovasc Diagn Ther 2016; 6: 138-43.
Maffessanti F, Gripari P, Tamborini G, et al. Evaluation of right ventricular systolic function after mitral valve repair: a two-dimensional Doppler, speckle-tracking, and three-dimensional echocardiographic study. J Am Soc Echocardiogr 2012; 25: 701-8.
Lindqvist P, Holmgren A, Zhao Y, Henein MY. Effect of pericardial repair after aortic valve replacement on septal and right ventricular function. Int J Cardiol 2012; 155: 388-93.
Unsworth B, Casula RP, Yadav H, et al. Contrasting effect of different cardiothoracic operations on echocardiographic right ventricular long axis velocities, and implications for interpretation of post-operative values. Int J Cardiol 2013; 165: 151-60.
Kempny A, Diller GP, Kaleschke G, et al. Impact of transcatheter aortic valve implantation or surgical aortic valve replacement on right ventricular function. Heart 2012; 98: 1299-304.
Alam M, Hedman A, Nordlander R, Samad B. Right ventricular function before and after an uncomplicated coronary artery bypass graft as assessed by pulsed wave Doppler tissue imaging of the tricuspid annulus. Am Heart J 2003; 146: 520-6.
Hedman A, Alam M, Zuber E, Nordlander R, Samad BA. Decreased right ventricular function after coronary artery bypass grafting and its relation to exercise capacity: a tricuspid annular motion-based study. J Am Soc Echocardiogr 2004; 17: 126-31.
Rosner A, Avenarius D, Malm S, et al. Changes in right ventricular shape and deformation following coronary artery bypass surgery-insights from echocardiography with strain rate and magnetic resonance imaging. Echocardiography 2015; 32: 1809-20.
David JS, Tousignant CP, Bowry R. Tricuspid annular velocity in patients undergoing cardiac operation using transesophageal echocardiography. J Am Soc Echocardiogr 2006; 19: 329-34.
Vogel M, Schmidt MR, Kristiansen SB, et al. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation 2002; 105: 1693-9.
Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: I. anatomy, physiology, and assessment. Anesth Analg 2009; 108: 407-21.
Silverton N, Meineri M. Speckle tracking strain of the right ventricle: an emerging tool for intraoperative echocardiography. Anesth Analg 2017. DOI:10.1213/ANE.0000000000001910.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28(1-39): e14.
Lindstrom L, Wigstrom L, Dahlin LG, Aren C, Wranne B. Lack of effect of synthetic pericardial substitute on right ventricular function after coronary artery bypass surgery. An echocardiographic and magnetic resonance imaging study. Scand Cardiovasc J 2000; 34: 331-8.
Pinto FJ, Wranne B, St Goar FG, et al. Systemic venous flow during cardiac surgery examined by intraoperative transesophageal echocardiography. Am J Cardiol 1992; 69: 387-93.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Charles J. Bitcon and Claude Tousignant contributed to study design, data collection, data analysis and drafting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bitcon, C.J., Tousignant, C. The effect of pericardial incision on right ventricular systolic function: a prospective observational study. Can J Anesth/J Can Anesth 64, 1194–1201 (2017). https://doi.org/10.1007/s12630-017-0972-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-0972-3