Abstract
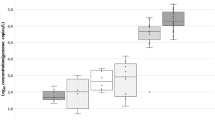
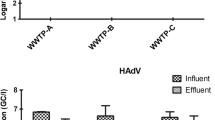
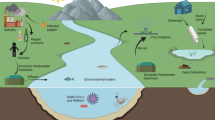
The Yucatan Peninsula of Mexico hosts a karst aquifer system that is the only source of freshwater for the area; however, it is vulnerable to human-mediated contamination. Pepper mild mottle virus (PMMoV) is one of the most abundant RNA viruses associated with human feces, making it a viable indicator for tracking fecal pollution in aquatic environments, including groundwater. In this study, groundwater samples collected from a karst aquifer from fresh and brackish water locations were analyzed for fecal indicator bacteria, somatic and male F+ specific coliphages, and PMMoV during the rainy and dry seasons. Total coliform bacteria were detected at all sites, whereas Escherichia coli were found at relatively low levels <40 MPN/100 ml. The highest average concentrations of somatic and male F+ specific coliphages were 920 and 330 plaque forming units per 100 ml, respectively, detected in freshwater during the rainy season. PMMoV RNA was detected in 85% of the samples with gene sequences sharing 99–100% of nucleotide identity with PMMoV sequences available in GenBank. Quantification of PMMoV genome copies (GC) by quantitative real-time PCR indicated concentrations ranging from 1.7 × 101 to 1.0 × 104 GC/L, with the highest number of GC detected during the rainy season. No significant correlation was observed between PMMoV occurrence by season or water type (p > 0.05). Physicochemical and indicator bacteria were not correlated with PMMoV concentrations. The abundance and prevalence of PMMoV in the karst aquifer may reflect its environmental persistence and its potential as a fecal indicator in this karst aquifer system.


Similar content being viewed by others
References
Agulló-Barceló, M., Galofré, B., Sala, L., García-Aljaro, C., Lucena, F., & Jofre, J. (2016). Simultaneous detection of somatic and F-specific coliphages in different settings by Escherichia coli strain CB390. FEMS Microbiology Letters, 363(17), 1–5.
Anderson, M. J., Gorley, R. N., & Clarke, K. R. (2008). PERMANOVAþ for PRIMER: Guide to software and statistical methods. Plymouth: PRIMER-E.
Arcega-Cabrera, F., Velázquez-Tavera, N., Fargher, L., Derrien, M., & Noreña-Barroso, E. (2014). Fecal sterols, seasonal variability, and probable sources along the ring of cenotes, Yucatan, Mexico. Journal of Contaminant Hydrology, 168, 41–49.
Bauer-Gottwein, P., Gondwe, B. R. N., Charvet, G., Marin, L. E., Rebolledo-Vieyra, M., & Merediz-Alonso, G. (2011). Review: The Yucatan Peninsula karst aquifer, Mexico. Hydrogeology Journal, 19, 507–524.
Betancourt, W. Q., Kitajima, M., Wing, A. D., Regnery, J., Drewes, J. E., Pepper, I. L., et al. (2014). Assessment of virus removal by managed aquifer recharge at three full-scale operations. Journal of Environmental Science and Health, 49(14), 1685–1692.
CE-CCA-001/89 Criterios Ecológicos de Calidad del Agua. Diario Oficial de la Federación. SEMARNAT, Comisión del Agua, 14 de diciembre de 1989. http://legismex.mty.itesm.mx/acu/acca001.pdf.
Comisión de Agua Potable y Alcantarillado del Estado de Quintana Roo. (2011). Programa de desarrollo institucional de infraestructura hidráulica y sanitaria 2011-2016. México: CAPA.
Derrien, M., Arcega, C. F., Velazquez, T. N. L., Kantún, M. C. A., & Capella, V. S. (2015). Sources and distribution of organic matter along the Ring Cenotes, Yucatan, Mexico: Sterol markers and statistical approaches. Science of Total Environment, 511, 223–229.
Encuesta Intercensal. (2015). Principales resultados de la Encuesta Intercensal 2015: Quintana Roo/Instituto Nacional de Estadística y Geografía. México: INEGI, c2015.
Fong, T.-T., & Lipp, E. K. (2005). Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews, 69(2), 357–371.
Gerba, C. P., Goyal, S. M., Labelle, R. L., Cech, I., & Bodgan, G. F. (1979). Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. American Journal of Public Health, 69(11), 1116–1119.
Hamza, I. A., Jurzik, L., Überla, K., & Wilhelm, M. (2011). Evaluation of pepper mild mottle virus, human picobirnavirus and Torque teno virus as indicators of fecal contamination in river water. Water Research, 45, 1358–1368.
Haramoto, E., Kitajima, M., Kishida, N., Konno, Y., Katayama, H., Asami, M., et al. (2013). Ocurrence of Pepper Mild Mottle Virus in drinking water sources in Japan. Applied and Environmental Microbiology, 79(23), 7413–7418.
Harwood, V. J., Boehmb, A. B., Sassoubreb, L. M., Vijayavelc, K., Stewartd, J. R., Fonge, T.-T., et al. (2013). Performance of viruses and bacteriophages for fecal source determination in a multi-laboratory, comparative study. Water Research, 47(18), 6929–6943.
Harwood, V. J., Staley, C., Badgley, B. D., Borges, K., & Korajkic, A. (2014). Microbial source tracking markers for detection of fecal contamination in environmental waters: Relationships between pathogens and human health outcomes. FEMS Microbiology Reviews, 38(1), 1–40.
Hernández-Morga, J., Leon, F. J., Peraza, G. F., Gil, S. B. G., & Chaidez, C. (2009). Detection and characterization of Hepatitis A Virus and Norovirus present in estuarine water samples using ultrafiltration- RT-PCR integrated methods. Journal of Applied Microbiology, 106(5), 1579–1590.
Hernández-Terrones, L. M., Null, K. A., Ortega-Camacho, D., & Paytan, A. (2015). Water quality assessment in the Mexican Caribbean: Impacts on the coastal ecosystem. Continental Shelf Research, 102, 62–72.
Hernández-Terrones, L., Rebolledo-Vieyra, M., Merino-Ibarra, M., Soto, M., Le-Cossec, A., & Monroy-Ríos, E. (2011). Groundwater Pollution in a Karstic Region (NE Yucatan): Baseline Nutrient Content and Flux to Coastal Ecosystems. Water, Air, and Soil Pollution, 218, 517–528.
Hill, V. R., Polaczyk, A. L., Hahn, D., Narayanan, J., Cromeans, T. L., Roberts, J., et al. (2005). Development of a rapid method for simultaneous recovery of diverse microbes in drinking water by ultrafiltration with sodium polyphosphate and surfactants. Applied and Environmental Microbiology, 71, 6878–6884.
Hunt, R. J., Borchardt, M. A., & Bradbury, K. R. (2014). Viruses as groundwater tracers: Using ecohydrology to characterize short travel times in aquifers. Ground Water, 52(2), 187–193.
Ikner, L. A., Soto-Beltran, M., & Bright, K. R. (2011). New method using a positively charged microporous filter and ultrafiltration for concentration of viruses from tap water. Applied and Environmental Microbiology, 77(10), 3500–3506.
Jofre, J., Lucena, F., Blanch, A. R., & Muniesa, M. (2016). Coliphages as model organisms in the characterization and management of water resources. Water. doi:10.3390/w8050199.
Johnson, T. B., McKay, L. D., Layton, A. C., Jones, S. W., Johnson, G. C., Cashdollar, J. L., et al. (2011). Viruses and bacteria in Karst and fractured rock aquifers in East Tennessee, USA. Ground Water, 49(1), 98–110.
Kitajima, M., Iker, B. C., Pepper, I. L., & Gerba, C. P. (2014). Relative abundance and treatment reduction of viruses during wastewater treatment processes—Identification of potential viral indicators. Science of Total Environment, 488–489(290), 296.
Kuroda, K., Nakada, N., Hanamoto, S., Inaba, M., Katayama, H., To, A. T., et al. (2015). Pepper mild mottle virus as an indicator and a tracer of fecal pollution in water environments: Comparative evaluation with wastewater-tracer pharmaceuticals in Hanoi, Vietnam. Science of the Total Environment, 506–507, 287–298.
La Rosa, G., Pourshaban, M., Iaconelli, M., & Muscillo, M. (2010). Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Annali dell’Istituto Superiore di Sanità, 46(3), 266–273.
Leal-Bautista, R. M., Hernández-Zárate, G., Jaime, N. A., Cuevas, G., & Velázquez, G. O. (2011). Pathogens and pharmaceuticals pollutants as indicators of contamination at the Northeasthern aquifer of Quintana Roo. Tropical and Subtropical Agroecosystems, 13, 211–219.
Leal-Bautista, R. M., Lenczewski, M., Morgan, C., Gabala, A., & McLain, J. E. (2013). Assesing fecal contamination in groundwater from the Tulum region, Quintana Roo, Mexico. Journal of Environmental Protection, 4, 1272–1279.
Lee, G. C., Jheong, W. H., Kim, M. J., Choi, D. H., & Baik, K.-H. (2013). A 5-year survey (2007-2011) of enteric viruses in Korean aquatic environments and the use of coliforms as viral indicators. Microbiology and Immunology, 57(1), 46–53.
Mahler, B. J., Personné, J.-C., Lods, G. F., & Drogue, C. (2000). Transport of free and particulate—Associated bacteria in karst. Journal of Hydrology, 238, 179–193.
Marin, L. E., Steinich, B., Pacheco, J., & Escolero, O. A. (2000). Hydrogeology of a contaminated sole-source karst aquifer, Merida, Yucatan, Mexico. Geofísica Internacional, 39, 359–365.
Medina-Moreno, S. A., Jiménez-González, A., Gutiérrez-Rojas, M., & Lizardi-Jiménez, M. A. (2014). Hydrocarbon pollution studies of underwater sinkholes along Quintana Roo as a function of tourism development in the mexican Caribbean. Revista Mexicana de Ingeniería Química, 13(2), 509–516.
Mehle, N., & Ravnikar, M. (2012). Plant viruses in aqueous environment—Survival, water mediated transmission and detection. Water Research, 46, 4902–4917.
Metcalfe, C. D., Beddows, P. A., Bouchot, G. G., Metcalfe, T. L., Li, H., & Van Lavieren, H. (2011). Contaminants in the coastal karst aquifer system along the Caribbean coast of the Yucatan Peninsula, Mexico. Environmental Pollution, 159(4), 991–997.
Munro, P. G., & Melo-Zurita, M. L. (2011). The role of cenotes in the social history of Mexico´s Yucatan Peninsula. Environment and History, 17, 583–612.
Pacheco, A. J., Cabrera, A. S., & Marín, L. E. (2000). Bacteriological contamination in the karstic aquifer of Yucatán Mexico. Geofísica Internacional, 39(3), 285–291.
Pacheco, A. J., Cabrera, A. S., & Pérez, C. R. (2004). Diagnóstico de la calidad del agua subterránea en los sistemas municipales de abastecimiento en el estado de Yucatán, México. Ingeniería, 8–2, 165–179.
Peng, J., Shi, B., Zheng, H., Lu, Y., Lin, L., Jiang, T., et al. (2015). Detection of pepper mild mottle virus in pepper sauce in China. Archives of Virology, 160, 2079–2082.
Pérez, L., Bugja, R., Lorenschat, J., Brenner, M., Curtis, J., Hoelzmann, P., et al. (2011). Aquatic ecosystems of the Yucatán Peninsula (Mexico), Belize, and Guatemala. Hydrobiologia, 661, 407–433.
Plummer, J. D., Sharon, C. L., Charest, A. J., & Roop, D. O. (2014). Bacterial and viral indicators of fecal contamination in drinking water. Journal American Water Works Association, 106(4), 200–211.
Polanco, R. A. G., Navarro, A. J. A., Solorio, S. J., Mena, R. G. J., Gómez, M. J., & Del Valls, C. T. A. (2015). Contamination by organochlorine pesticides in the aquifer of the Ring of Cenotes in Yucatán, México. Water and Environment Journal, 29, 140–150.
Reed, T. M., Fryar, A. E., Brion, G. M., & Ward, J. W. (2011). Differences in pathogen indicators between proximal urban and rural karst springs, Central Kentucky, USA. Environmental Earth Sciences, 64, 47–55.
Rodríguez-Martínez, R. E. (2008). Community involvement in marine protected areas: The case of Puerto Morelos reef, México. Journal of Environmental Management, 88, 1151–1160.
Rosario, K., Symonds, E. M., Sinigalliano, C., Stewart, J., & Breitbart, M. (2009). Pepper mild mottle virus as an indicator of fecal pollution. Applied and Environmental Microbiology, 75, 7261–7267.
Sambrook, J., Rusell, D. W., & Cold Spring Harbor Laboratory. (2001). Molecular cloning: A laboratory manual. New York: Cold Spring Harbor Laboratory.
Schiperski, F., Zirlewagen, J., & Scheytt, T. (2016). Transport and attenuation of particles of different density and surface charge: A karst aquifer field study. Environmental Science and Technology, 50(15), 8028–8035.
Schmitter-Soto, J. J., Comín, F. A., Escobar-Briones, E., Herrera-Silveira, J., Alcocer, J., Suárez-Morales, E., et al. (2002). Hydrogeochemical and biological characteristics of cenotes in the Yucatan Peninsula (SE Mexico). Hydrobiologia, 467, 215–228.
Schmitz, B. W., Kitajima, M., Campillo, M. E., Gerba, C. P., & Pepper, I. L. (2016). Virus reduction during advanced bardenpho and conventional wastewater treatment processes. Environmental Science and Technology, 50, 9524–9532.
Sinreich, M., Pronk, M., & Kozel, R. (2014). Microbiological monitoring and classification of karst springs. Environmental Earth Sciences, 71, 563–572.
Symonds, E. M., Sinigalliano, C., Gidley, M., Ahmed, W., McQuaig-Ulrich, S. M., & Breitbart, M. (2016). Faecal pollution along the southeastern coast of Florida and insight into the use of pepper mild mottle virus as an indicator. Journal of Applied Microbiology, 121, 1469–1481.
Updyke, E. A., Wang, Z., Sun, S., Connell, C., Kirs, M., Wong, M., et al. (2015). Human enteric viruses—Potential indicators for enhanced monitoring of recreational water quality. Virologica Sinica, 30(5), 344–353.
U.S. EPA. (2015). Review of coliphages as possible indicators of fecal contamination for ambient water quality. EPA 820-R-15-098. Washington, DC: Office of Water, Science and Technology Health and Ecological Criteria Division. https://www.epa.gov/sites/production/files/2016-07/documents/review_of_coliphages_as_possible_indicators_of_fecal_contamination_for_ambient_water_quality.pdf.
Williamson, K. E., Harris, J. V., Green, J. C., Rahman, F., & Chambers, R. M. (2014). Stormwater runoff drives viral community composition changes in inland freshwaters. Frontiers Microbiology. doi:10.3389/fmicb.2014.00105.
Zar, J. H. (1996). Biostatistical analysis. Upper Saddle River: Prentice Hall.
Zhang, T., Breitbart, M., Lee, W. H., Run, J.-Q., Wei, C. L., Soh, S. W. L., et al. (2006). RNA viral community in human feces: Prevalence of plant pathogenic viruses. PLoS Biology, 4(1), e3.
Acknowledgements
We wish to thank undergraduate students, Ramón Vega Vázquez, Hans Cristian Basilio Cortés, Guadalupe del Carmen Albornoz Negroe, and Delia Maleny Chan Correa for their assistance in obtaining PMMoV sequences. We also extend our special thanks to Dr. Gerardo López and M.C. Francisco Javier García Villalobos for their technical assistance with qPCR. Also we acknowledge the many anonymous wastewater treatment plant personnel in the Riviera Maya, Quintana Roo, México for their cooperation and assistance with sample collections. Gerardo Ávila Torres was supported by a master degree scholarship (Number 575182) from Consejo Nacional de Ciencia y Tecnología (Conacyt). This project (Number 216093) was funded by Consejo Nacional de Ciencia y Tecnología (Conacyt)/“Proyectos de Desarrollo Científico para Atender Problemas Nacionales 2013.” The corresponding author wants to thank the Red Mexicana de Virología/Redes temáticas Conacyt, for a travel award.
Funding
This study was funded by Grant Number 216093 by Consejo Nacional de Ciencia y Tecnología (Conacyt)/“Proyectos de Desarrollo Científico para Atender Problemas Nacionales 2013.” Gerardo Ávila Torres was supported by a master degree scholarship Number 575182 from Consejo Nacional de Ciencia y Tecnología (Conacyt). Cecilia Hernández Zepeda received a travel award from the Red Mexicana de Virología.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rosiles-González, G., Ávila-Torres, G., Moreno-Valenzuela, O.A. et al. Occurrence of Pepper Mild Mottle Virus (PMMoV) in Groundwater from a Karst Aquifer System in the Yucatan Peninsula, Mexico. Food Environ Virol 9, 487–497 (2017). https://doi.org/10.1007/s12560-017-9309-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-017-9309-1