Abstract
Backgrounds
Takotsubo syndrome (TTS) is an intriguing clinical entity characterized by transient myocardial dysfunction. The precise pathophysiological mechanism of TTS is still unknown, but recent evidence suggests a central role of systemic inflammation associated with adrenergic discharge. Although initially considered benign, TTS has shown several potential short-term and long-term complications and adverse outcomes. To improve understanding and management, advanced cardiovascular magnetic resonance (CMR) techniques, such as feature tracking (FT) and parametric mapping, have gained attention.
Purpose of Review
The purpose of this review is to summarize the current literature on the clinical applications of CMR-FT and mapping in TTS. Additionally, the most significant and recent findings will be discussed.
Recent Findings
FT-CMR enables the parametric quantification of myocardial deformation, allowing a comprehensive evaluation of left ventricular, right ventricular, and atrial function. It provides an accurate definition of areas of myocardial dysfunction and potentially serves as a superior prognostic tool compared to ejection fraction. Tissue mapping techniques enable precise and comprehensive tissue characterization by quantifying areas of oedema, and myocardial fibrosis.
Summary
FT-CMR and mapping techniques serve as valuable prognostic tools both in the acute and chronic phases of TTS. They can detect subtle alterations and pan-cardiac involvement, while also providing important insights into the complex underlying mechanisms of the syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Takotsubo syndrome (TTS) is a cardiac disorder characterized by transient myocardial dysfunction that mimics acute myocardial infarction [1]. Despite initially considered a benign condition [2, 3], growing evidence suggests that the prognosis of TTS is not always favourable, with potential complications such as heart failure, cardiogenic shock, and death [4,5,6,7,8,9]. In fact, some studies have reported that TTS carries a similar or even higher risk of adverse outcomes compared to acute coronary syndrome [4, 10,11,12,13]. Importantly, this unfavourable prognosis extends beyond the acute phase [14,15,16]. This highlights the need for better understanding of the pathophysiology of TTS and the development of more effective management strategies.
The diagnosis of TTS is based on clinical, electrocardiographic, and echocardiographic findings. However, the role of cardiovascular magnetic resonance (CMR) imaging in the diagnosis and management of TTS has been increasingly recognized in recent years [17]. CMR is considered the gold standard for the evaluation of myocardial structure and function. In the context of TTS, it is recommended to perform CMR during the acute phase whenever possible. This allows for a comprehensive assessment of the disease providing useful clinical information for patient management [18]. New CMR techniques are continuously emerging and finding applications in many clinical settings, thanks to advances in related software and hardware techniques. Two techniques that have garnered considerable interest in recent years are CMR feature-tracking (CMR-FT) for myocardial strain analysis and parametric T1 and T2 mapping for myocardial tissue characterization.
In the present review, we will summarize and discuss the most recent findings regarding CMR-FT and mapping in TTS. Additionally, we will explore their significance and potential clinical applications.
Cardiovascular Magnetic Resonance Feature Tracking
Myocardial strain, also known as myocardial deformation imaging, is a technological advancement that has been developed to objectively quantify regional myocardial function beyond ejection fraction [19, 20].
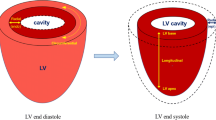
It is a dimensionless index of the length change between two given points, reflecting the degree of myocardial deformation. The formula to calculate strain (ε) is (L − L0)/L0, where L0 is the baseline length (usually end-diastole), and L is the instantaneous length at the time of measurement (usually at end-systole). The amount of deformation is expressed in percentages. Negative strain means shortening, thinning, and contraction, while positive strain means lengthening, thickening, and relaxation. Myocardial mechanics can be investigated in all directions of myocardial movement: longitudinal shortening, circumferential shortening, and radial thickening. Initially, echocardiography was utilized to evaluate myocardial deformation. However, over time, several CMR techniques have been introduced as alternative approaches. These include myocardial tagging, displacement encoding, and strain encoded imaging. Each technique has its limitations, such as the need for additional acquisitions, low signal-to-noise ratio, lack of standardization, and demanding post-processing requirements [21,22,23]. FT-CMR is a novel technique that uses a block-matching approach to identify anatomic features in CMR images along the myocardial boundaries and track them along the cardiac cycle by searching for the most comparable image pattern in the successive image [24, 25]. In many previous studies, FT-CMR has demonstrated excellent reproducibility and good correlation with tagging [26,27,28]. Compared to CMR tagging, CMR-FT is easier to perform without the need for dedicated acquisition and complex post-processing, as it can be applied to standard CMR cine Steady State Free Precession (SSFP) sequences, and it is highly reproducible [29, 30]. Thus, FT-CMR potentially has an important role in TTS because acute myocardial dysfunction is a prominent feature of the condition and the extent of dysfunction is one of the major prognostic factors in the short and long term [7]. Moreover involvement can extend beyond the distinctly akinetic areas of the left ventricle (LV) [31], affecting both the right ventricle (RV) [32] and left atrium (LA) [33].
Left Ventricle Strain
Assessing LV dysfunction in patients with TTS is crucial for accurate diagnosis and management. Hypokinesia, akinesia, or dyskinesia of the apical, midventricular, and basal segments are common patterns of LV dysfunction observed in TTS patients. While the apical ballooning pattern is the most recognizable and frequently observed, atypical patterns such as focal TTS can be more challenging to identify and diagnose using standard cardiovascular imaging methods [4, 34]. By detecting subtle abnormalities and accurately quantifying the extent of myocardial dysfunction, CMR-FT provides a more comprehensive and detailed evaluation of ventricular performance. During the acute phase of TTS, segmental analyses of peak circumferential and peak longitudinal strain provide objective assessments of regional LV contraction abnormalities, enabling discrimination of different ballooning patterns [35•]. Furthermore, FT-CMR evaluation of LV rotational mechanics reveals transient dyssynchrony, particularly pronounced in the subset of TTS patients with stressful triggers, comorbidities, and higher mortality risk [36•].
The extent of myocardial dysfunction has a well-established role in short- and long-term prognoses for TTS patients [37, 38]. Notably, typical apical ballooning is associated with more pronounced alterations of global circumferential and longitudinal strain and is linked to more severe LV dysfunction and increased mortality [35•, 38]. Stiermaier and colleagues have shown that strain values, including global circumferential, longitudinal, and radial strain, are significantly lower in TTS patients than in healthy subjects or in those with non-ST-segment elevation myocardial infarction (NSTEMI), but similar to those with ST-segment elevation myocardial infarction (STEMI) [35•]. In clinical practice, measurement of ejection fraction (EF) is the most popular method for assessing ventricular performance. However, in TTS, EF is often only moderately reduced since regional hypercontraction balances pronounced wall motion abnormalities. As a result, EF may not adequately reflect the extent of LV systolic dysfunction. Deformation indices, such as CMR-FT-derived strain, may provide superior prognostic markers in TTS. In this regard, LV longitudinal strain has emerged as a superior clinical outcome marker when compared to EF in a group of TTS patients. Specifically, observed long-term mortality rates were significantly higher in patients with FT-derived global longitudinal strain values greater than about -11% [39•]. Moreover, while myocardial dysfunction in TTS was previously believed to be entirely reversible, recent studies have shown that patients may experience long-term echocardiographic wall motion abnormalities, despite apparent recovery of EF [40••].
Right Ventricle Strain
Right ventricular (RV) involvement has been observed in a significant number of TTS patients and has been associated with worse outcomes, including prolonged hospitalization and increased short- and long-term adverse events [32, 41,42,43]. Typically, RV wall motion abnormalities are concentrated in the apical segments, while mid and basal RV contraction remains preserved or hyperkinetic [44•].
The visualization and assessment of the extent of regional abnormalities in the RV are challenging with echocardiography due to its complex geometry. Similar to the left ventricle, hypercontractile segments may partially compensate for dysfunctional segments, resulting in a less sensitive detection of the extent of RV systolic dysfunction through EF measurements. FT-CMR evaluation of longitudinal RV strain has been shown to outperform subjective visual assessment of RV involvement [44•]. Additionally, it has demonstrated a correlation between LVEF and long-term risk stratification, enabling more accurate identification of high-risk individuals [32, 44•, 45]. Patients with biventricular involvement may be particularly prone to a severe clinical course with heart failure and/or cardiogenic shock due to the higher degree of LV dysfunction, which is further compromised by reduced LV preload resulting from RV dysfunction. However, further studies are needed to determine if these hemodynamic alterations can impact long-term prognosis.
Atrial Strain
Although the most obvious manifestations in TTS are seen in the LV, atrial involvement has also been described [33]. FT-CMR allows for the evaluation of both left and right atrial performance by quantifying the atrial reservoir, conduit, and booster functions based on absolute strain values and corresponding strain rates [46]. Reservoir function refers to the collection of pulmonary venous return in the atrium during ventricular systole, while conduit function represents the early diastolic blood passage during ventricular filling. Booster pump function is responsible for the late diastolic augmentation of ventricular filling. During the acute phase of TTS, both LA reservoir function and LA conduit function are significantly impaired and tend to recover completely at follow-up [47•, 48]. In contrast, LA booster pump function is normal or even increased within the acute phase [47•]. It is likely that LA reservoir function and conduit function are reduced due to increased LV filling pressures resulting from LV diastolic dysfunction. Increased LA contractility, along with prolonged myocardial relaxation, may be a compensatory response to decreased LV diastolic filling, and helps maintain adequate antegrade flow. A recent invasive hemodynamic study has indeed demonstrated severe diastolic dysfunction of the left ventricle in patients with TTS compared to a control group without cardiovascular diseases. Specifically, the invasive assessment of pressure–volume loops revealed prolonged myocardial active relaxation (an energy-dependent process) and normal ventricular compliance (passive ventricular filling) [49]. Further studies are needed to better understand the intricate hemodynamic alterations responsible for diastolic dysfunction in TTS.
A recent study investigating LA strain using FT-CMR demonstrated that impaired LA function during the acute phase was associated with long-term mortality, independent of traditional cardiovascular risk factors and LVEF [47•].
Table 1 summarizes recent findings on FT-CMR and provides an interpretation of its clinical utility.
In summary, FT-CMR has emerged as a promising tool for the assessment of TTS patients, providing an accurate and comprehensive evaluation of myocardial function. Specifically, FT-CMR allows for the detection of global myocardium involvement, even beyond areas of visually assessed abnormal wall motion. This has important clinical implications for the diagnosis, management, and long-term risk stratification of TTS patients. While further studies are needed to fully establish the role of FT-CMR in TTS, the current evidence suggests that it has great potential in improving our understanding and management of this complex syndrome.
Parametric Mapping
Accurate non-invasive tissue characterization is crucial for the diagnosis and management of TTS as it enables differentiation from other conditions. Moreover, it provides valuable insights into the underlying mechanisms of TTS.
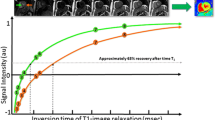
In recent years, advanced techniques have been continuously developing to enhance the assessment and quantification of myocardial tissue properties. One of these techniques is mapping, which entails the acquisition of a series of images using various contrast weightings or imaging parameters, such as T1, T2, or T2* relaxation times. By analysing the signal intensity values in these images, parametric maps can be generated, which provide both qualitative (Fig. 1) and quantitative (Fig. 2) information about tissue characteristics, such as myocardial fibrosis or oedema. The signal intensity primarily depends on extracellular water content (T2 mapping), as well as fibrosis and infiltration of fat or amyloid (native T1 and ECV).
Quantitative analyses of T2 mapping data in a patient with TTS. A progressive increase in T2 mapping values is observed from basal to apical segments. Of note, subtle myocardial edema is detected at basal segments too as indicated by border-line increase in T2 mapping value (50.5 ms, normal value for the scanner and sequence used is up to 50 ms)
Pathophysiology
Although the precise underlying mechanisms of TTS remains incompletely elucidated, recent studies using advanced imaging techniques have indicated a notable involvement of inflammation in its pathogenesis. Catecholaminergic surge during stress has been linked to the activation of pro-inflammatory cytokines, suggesting a possible connection between systemic inflammation and TTS [50]. Inflammatory conditions like infections or surgeries can further contribute to systemic inflammation and its association with TTS [51]. Catecholamines affect systemic inflammation through adrenoreceptors on inflammatory cells, influencing blood and lymph flow as well as the distribution of pro-inflammatory cells [52, 53]. Mechanistically, factors such as endotoxins and catecholaminergic discharge can trigger nitrosative stress, leading to increased myocardial vascular permeability, plasma leakage, and infiltration of pro-inflammatory cells, can result in myocardial oedema [54,55,56]. Of note, oedema remains a relatively non-specific marker of myocardial involvement that characterizes cardiac injury in the course of several diseases, including myocardial infarction [57], myocarditis [58], and cardiomyopathies [59,60,61]. However, oedema and associated inflammatory features may vary according to the underlying disease, potentially marking differences in the pathogenic processes; in example, neutrophils surge, proportional to the extent of the myocardial oedema, characterizes the acute phase of myocardial infarction [62], whereas lymphocytes or eosinophils infiltrates are more common in acute myocarditis [63].
In this context, a significant clinical study has provided compelling evidence for the presence and features of local myocardial inflammation in TTS patients. This study utilized multiparametric CMR with ultra-small superparamagnetic particles of iron oxide (USPIO) to detect and characterize myocardial inflammation. The USPIO enhancement was higher in patients with TTS compared to a matched control group in both ballooning and not-ballooning LV myocardial segments. This increased enhancement indicated a significant infiltration of activated and phagocytic M1 type macrophages within the myocardial tissue of all TTS patients. Furthermore, patients with acute TTS showed changes in peripheral monocyte subsets and increased systemic levels of pro-inflammatory cytokines, providing further support for the involvement of systemic inflammation and its correlation with the inflammatory response within the myocardium [64••].
Recent studies have also investigated the role of epicardial fat in TTS. Epicardial fat is metabolically active visceral fat, and a recent CMR study has shown a correlation between the volume of epicardial fat and CMR markers of myocardial inflammation and subclinical contractile dysfunction in TTS patients [65].
CMR techniques have also been used to investigate alterations in myocardial metabolism and calcium handling in TTS. Acute impairment in energetic status [64••] and abnormal myocardial calcium handling [66] have been observed and can persist for at least three months, suggesting their involvement in the pathophysiology of the disease.
Overall, the integration of imaging techniques has provided valuable insights into the pathophysiology of TTS, highlighting the role of inflammation, metabolic abnormalities, and altered calcium handling.
Clinical and Prognostic Implications
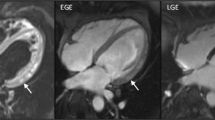
The typical CMR appearance of TTS is characterized by widespread myocardial oedema without significant replacement fibrosis observed in late gadolinium enhancement (LGE) imaging [31, 67]. However, recent studies have indicated that LGE may be present in some TTS patients [68, 69]. When LGE is detected, it is typically of low intensity, less than 5 standard deviations, suggesting minimal myocardial injury or necrosis [31]. In other cases, incidental findings from chronic and unrelated co-pathologies can be considered too [70].
More recently, studies using T1 and T2 mapping tools in TTS were published. In the acute phase of TTS, a significant increase in native T1, T2, and ECV has been observed [40••, 71, 72]. Non-contrast T1 and T2 mapping have demonstrated high diagnostic accuracy in identifying acute myocardial injury in patients with mid-apical TTS without the need of gadolinium contrast [73]. It is important to highlight that there is a direct correlation between T2, native T1, and ECV [73]. The reason behind this direct correlation could be explained by the significant impact of extracellular myocardial oedema on the interstitial space during the acute phase [74, 75•]. Specifically, extensive and widespread oedema not only affects T2 but also T1 mapping-derived measurements, including native T1 and ECV [76]. Hence, the rise of ECV in the acute phase of TTS is expression two simultaneous processes: extensive oedema and remodelling of the extracellular matrix. Moreover, increased myocardial water content and extracellular volume are observed not only in regions with abnormal wall motion but also, to a lesser extent, in areas with normal kinesis compared to controls [75•]. This finding suggests that myocardial involvement extends beyond regions with wall motion abnormalities, including the RV. The added clinical value of tissue mapping in this scenario has been highlighted by a recent prospective study of a cohort of patients with myocardial infarction with non-obstructive coronary arteries (MINOCA) who underwent early CMR imaging with T1 and ECV: compared to patients with MINOCA imaged using CMR without mapping techniques, a higher rate of TTS diagnosis was obtained [67].
Quantification of oedema using parametric techniques holds potential prognostic value, as its presence and extent have been associated with both electrocardiographic abnormalities and potential complications in TTS [75•, 77, 78]. Higher T2 values within the initial days following the acute event have been found in TTS patients with delayed recovery [72] and are inversely correlated with LV systolic function [75•]. Additionally, a larger difference in T2 values between the apex and base of the LV, indicative of higher oedema dispersion and gradient, is associated with more pronounced T-wave inversion and a longer QTc interval, potentially explaining the electrical instability observed in TTS patients [75•]. However, it is important to note that data regarding mortality in relation to CMR mapping findings in TTS are still limited due to the small sample sizes of current studies.
During the recovery phase, mapping techniques can detect persistent abnormalities despite complete normalization of systolic function. T2 mapping values tend to normalize over the follow-up period, although they may remain slightly elevated for approximately 3–5 months after the acute phase [79, 80]. On the other hand, T1 values may remain elevated for a longer period. In recovered TTS patients, native T1 in the LV has been found to be persistently elevated compared to a matched control group, even more than 1 year after the acute event. This observation is accompanied by impaired cardiac deformation (despite preserved LVEF), higher levels of natriuretic peptides, and persistent cardiac limitations observed during exercise testing at cardiopulmonary stress tests [40••]. Despite the well-known technical limitations in performing tissue mapping in thin free wall of the RV, it was also demonstrated a persistent increase in ECV values in the RV of patients with previous TTS [80]. These findings suggest the presence of subtle long-term non-transitory abnormalities that may partly explain the persistence of symptoms and unfavourable prognosis of TTS patients [40••]. Moreover, magnetic resonance imaging with ultra-small superparamagnetic iron oxide particles (USPIO) has shown signs of macrophage inflammatory infiltrate in the acute phase: though these changes regressed at a 5 months follow-up, signs of systemic inflammation persisted [64••]. Altogether, this supports the notion that persistent myocardial inflammation can be present even after the complete recovery of systolic function. According to this hypothesis, the long-term increase of certain biomarkers associated with inflammation, including BNP, has been demonstrated [50, 81, 82]. However, it cannot be excluded that such abnormalities were pre-existent the TTS attack, especially when considering the large comorbid burden that characterizes these patients [83].
The evaluation of interstitial fibrosis or ongoing inflammatory processes using mapping techniques appears potentially useful for prognostic stratification and guiding therapy, however, studies supporting these findings as well as clinical trials in this context are still limited.
Table 2 summarizes mapping techniques for TTS tissue characterization. Each technique is described along with its specific features. Additionally, the table includes the most significant findings and their clinical significance in different phases of the disease.
Conclusions
FT-CMR and mapping techniques have emerged as valuable tools for evaluating TTS, a complex clinical condition that extends beyond a benign and transient LV systolic dysfunction. These imaging techniques allow for the detection of subtle abnormalities, such as pan-cardiac involvement or mild alterations in regional systolic function, despite complete normalization of LVEF. Moreover, they provide insights into long-term myocardial abnormalities, including interstitial fibrosis or ongoing inflammation, potentially guiding therapeutic strategies during follow-up. However, there are certain limitations to consider, including variability in image acquisition and analysis, limited sample sizes, and technical challenges that impact the accuracy and clinical applicability of these modalities. Future directions involve standardization and multicentre collaborations to establish protocols and cut-off values, integration of novel imaging techniques [84], longitudinal studies for prognostic evaluation, and therapeutic monitoring for personalized medicine. By addressing these limitations and exploring these perspectives, the clinical utility of mapping and FT-CMR in TTS can be enhanced, leading to improved diagnosis, characterization, and patient management.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:1955–71.
Cacciotti L, Passaseo I, Marazzi G, Camastra G, Campolongo G, Beni S, Lupparelli F, Ansalone G. Observational study on Takotsubo-like cardiomyopathy: Clinical features, diagnosis, prognosis and follow-up. BMJ Open. 2012;2:1–6.
Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-Year Recurrence Rate and Prognosis of the Apical Ballooning Syndrome. J Am Coll Cardiol. 2007;50:448–52.
Templin C, Ghadri JR, Diekmann J, Napp C, Bataiosu D, Jaguszewski M, Cammann V, Sarcon A, Geyer V, Neumann C, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Cuneo A, Kuck K, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–38.
Arcari L, Limite LR, Cacciotti L, Sclafani M, Russo D, Passaseo I, Marazzi G, Ansalone G, Volpe M, Autore C, Musumeci MB. Admission heart rate and in-hospital course of patients with Takotsubo syndrome. Int J Cardiol. 2018;273:15–21.
Santoro F, Núñez Gil IJ, Stiermaier T, El-Battrawy I, Guerra F, Novo G, Guastafierro F, Tarantino N, Novo S, Mariano E, Romeo F, Romeo F, et al. Assessment of the German and Italian Stress Cardiomyopathy Score for Risk Stratification for In-hospital Complications in Patients With Takotsubo Syndrome. JAMA Cardiol. 2019;4:892–9.
Almendro-Delia M, Núñez-Gil IJ, Lobo M, Andrés M, Vedia O, Sionis A, Martin-García A, Cruz Aguilera M, Pereyra E, Martín de Miguel I, Linares Vicente JA, Corbí-Pascual M, et al. Short- and Long-Term Prognostic Relevance of Cardiogenic Shock in Takotsubo Syndrome: Results From the RETAKO Registry. JACC Heart Fail. 2018;6:928–36.
Santoro F, Ferraretti A, Ieva R, Musaico F, Fanelli M, Tarantino N, Scarcia M, Caldarola P, Di BM, Brunetti ND. American Journal of Emergency Medicine Renal impairment and outcome in patients with takotsubo cardiomyopathy. Am J Emerg Med. 2016;34:548–52.
Núñez-gil IJ, Almendro-delia M, Andrés M, Sionis A, Martin A, Bastante T, Juan G, García B, Villa D, Corbí-pascua M. Secondary forms of Takotsubo cardiomyopathy : A whole different prognosis. Eur Heart J Acute Cardiovasc Care. 2016;5:308–16.
Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C, Vonthein R, Schuler G, Thiele H, Eitel I. Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail. 2016;18:650–6.
Redfors B, Vedad R, Angerås O, Råmunddal T, Petursson P, Haraldsson I, Ali A, Dworeck C, Odenstedt J, Ioaness D, Libungan B, Shao Y, et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction — A report from the SWEDEHEART 1 registry. Int J Cardiol. 2015;185:282–9.
Vallabhajosyula S, Dunlay SM, Murphree DH, Barsness GW, Sandhu GS, Lerman A, Prasad A. Cardiogenic Shock in Takotsubo Cardiomyopathy Versus Acute Myocardial Infarction: An 8-Year National Perspective on Clinical Characteristics, Management, and Outcomes. JACC Heart Fail. 2019;7:469–76.
Scudiero F, Arcari L, Cacciotti L, De Vito E, Marcucci R, Passaseo I, Limite LR, Musumeci MB, Autore C, Citro R, Bossone E, Sanna GD, Bacchi B, Volpe M, Di Mario CPG. Prognostic relevance of GRACE risk score in Takotsubo syndrome. Euro Heart J Acute Cardiovasc Care. 2020;9(7):721–8.
Arcari L, Núñez Gil IJ, Stiermaier T, El-Battrawy I, Guerra F, Novo G, Musumeci B, Cacciotti L, Mariano E, Caldarola P, Parisi G, Montisci R, et al. Gender Differences in Takotsubo Syndrome. J Am Coll Cardiol. 2022;79:2085–93.
Sclafani M, Arcari L, Russo D, Tini G, Limite LR, Cacciotti L, Volpe M, Autore C, Musumeci MB. Long-term management of Takotsubo syndrome: A not-so-benign condition. Rev Cardiovasc Med. 2021;22:597–611.
Arcari L, Cacciotti L, Limite LR, Russo D, Sclafani M, Semeraro R, Ansalone G, Volpe M, Autore C, Musumeci MB. Clinical characteristics of patients with takotsubo syndrome recurrence: An observational study with long-term follow-up. Int J Cardiol. 2021;329:23–7.
Jensch PJ, Stiermaier T, Eitel I. Takotsubo Syndrome—Is There a Need for CMR? Curr Heart Fail Rep. 2021;18:200–10.
Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, et al. Current state of knowledge on Takotsubo syndrome : a position statement from the task force on Takotsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27.
McCulloch M, Gresser C, Moos S, Odabashian J, Jasper S, Bednarz J, Burgess P, Carney D, Moore V, Sisk E, Waggoner A, Witt S, et al. Ultrasound contrast physics: a series on contrast echocardiography, article 3. J Am Soc Echocardiogr. 2000;13:959–67.
Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography: Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–64.
Kihlberg J, Gupta V, Haraldsson H, Sigfridsson A, Sarvari SI, Ebbers T, Engvall JE. Clinical validation of three cardiovascular magnetic resonance techniques to measure strain and torsion in patients with suspected coronary artery disease. J Cardiovasc Magn Reson. 2020;22:83.
Osman NF, Sampath S, Atalar E, Prince JL. Imaging longitudinal cardiac strain on short-axis images using strain-encoded MRI. Magn Reson Med. 2001;46:324–34.
Voigt JU, Cvijic M. 2- and 3-dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. 2019;12:1849–63.
Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016;18:51.
Claus P, Omar AMS, Pedrizzetti G, Sengupta PP, Nagel E. Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging. 2015;8:1444–60.
Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:1.
Nazir SA, Shetye AM, Khan JN, Singh A, Arnold JR, Squire I, McCann GP. Inter-study repeatability of circumferential strain and diastolic strain rate by CMR tagging, feature tracking and tissue tracking in ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging. 2020;36:1133–46.
Augustine D, Lewandowski AJ, Lazdam M, Rai A, Francis J, Myerson S, Noble A, Becher H, Neubauer S, Petersen SE, Leeson P. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: Comparison with tagging and relevance of gender. J Cardiovasc Magn Reson. 2013;15:8.
Truong VT, Palmer C, Wolking S, Sheets B, Ngo TNM, Taylor M, Nagueh SF, Zareba KM, Raman S, Mazur W. Normal left atrial strain and strain rate using cardiac magnetic resonance feature tracking in healthy volunteers. Eur Heart J Cardiovasc Imaging. 2020;21:446–53.
Bucius P, Erley J, Tanacli R, Zieschang V, Giusca S, Korosoglou G, Steen H, Stehning C, Pieske B, Pieske-kraigher E, Schuster A, Lapinskas T, et al. Comparison of feature tracking, fast-SENC, and myocardial tagging for global and segmental left ventricular strain. ESC Heart Fail. 2019;7:523–32.
Eitel I, Von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA - J Am Med Assoc. 2011;306:277–86.
Kagiyama N, Okura H, Tamada T, Imai K, Yamada R, Kume T, Hayashida A, Neishi Y, Kawamoto T, Yoshida K. Impact of right ventricular involvement on the prognosis of takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17:210–6.
Stiermaier T, Graf T, Möller C, Eitel C, Ledwoch J, Desch S, Gutberlet M, Schuler G, Thiele H, Eitel I. Transient left atrial dysfunction is a feature of Takotsubo syndrome. J Cardiovasc Magn Reson. 2017;19:15.
Tini G, Limite LR, Arcari L, Cacciotti L, Russo D, Sclafani M, Brunelli C, Volpe M, Autore C, Musumeci MB. A systematic review on focal takotsubo syndrome: a not-so-small matter. Heart Fail Rev. 2022;27:271–80.
• Stiermaier T, Lange T, Chiribiri A, Möller C, Graf T, Raaz U, Villa A, Kowallick JT, Lotz J, Hasenfuß G, Thiele H, Schuster A, et al. Left ventricular myocardial deformation in Takotsubo syndrome: a cardiovascular magnetic resonance myocardial feature tracking study. Eur Radiol. 2018;28:5160–70. This FT-CMR study has demonstrated that global circumferential, longitudinal, and radial strain values assessed in the acute phase are significantly lower in TTS patients compared to healthy subjects or individuals with NSTEMI, but similar to those with STEMI.
• Backhaus SJ, Stiermaier T, Lange T, Chiribiri A, Lamata P, Uhlig J, Kowallick JT, Raaz U, Villa A, Lotz J, Hasenfuß G, Thiele H, et al. Temporal changes within mechanical dyssynchrony and rotational mechanics in Takotsubo syndrome: A cardiovascular magnetic resonance imaging study. Int J Cardiol. 2018;273:256–62. This FT-CMR study evaluating LV rotational mechanics in TTS patients has revealed transient dyssynchrony, which is particularly pronounced in the subset of TTS patients with stressful triggers, comorbidities, and higher mortality risk.
Ghadri J, Cammann V, Napp C, Jurisic S, Diekmann J, Bataiosu D, Seifert B, Jaguszewski M, Sarcon A, Neumann C, Geyer V, Prasad A, et al. Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome data from the international takotsubo registry. JAMA Cardiology. 2016;1:335–40.
Stiermaier T, Möller C, Graf T, Eitel C. Prognostic Usefulness of the Ballooning Pattern in Patients With Takotsubo Cardiomyopathy. Am J Cardiol. 2016;118:1737–41.
• Stiermaier T, Busch K, Lange T, Pätz T, Meusel M, Backhaus SJ, Frydrychowicz A, Barkhausen J, Gutberlet M, Thiele H, Schuster A, Eitel I. Prognostic value of different CMR-based techniques to assess left ventricular myocardial strain in Takotsubo syndrome. J Clin Med. 2020;9:1–13. This comprehensive FT-CMR study demonstrated that a global longitudinal strain value higher than approximately -11% in the acute phase identifies patients at a high long-term risk.
•• Scally C, Rudd A, Mezincescu A, Wilson H, Srivanasan J, Horgan G, Broadhurst P, Newby DE, Henning A, Dawson DK. Persistent long-term structural, functional, and metabolic changes after stress-induced (takotsubo) cardiomyopathy. Circulation. 2018;137:1039–48. This study demonstrated persistently increased native T1 values in TTS patients compared to a matched control group after more than one year from the acute event, in association with impaired cardiac deformation (despite preserved LVEF), higher levels of natriuretic peptides, and persistent cardiac limitations observed during exercise testing at cardiopulmonary stress tests.
Fitzgibbons TP, Madias C, Seth A, Bouchard JL, Kuvin JT, Patel AR, Pandian NG, Meyer TE, Aurigemma GP, Tighe DA. Prevalence and Clinical Characteristics of Right Ventricular Dysfunction in Transient Stress Cardiomyopathy. AJC. 2009;104:133–6.
Becher T, El-battrawy I, Baumann S, Fastner C, Behnes M, Loßnitzer D, Elmas E, Hoffmann U, Papavassiliu T, Kuschyk J, Dösch C, Röger S, et al. Characteristics and long-term outcome of right ventricular involvement in Takotsubo cardiomyopathy. Int J Cardiol. 2016;220:371–5.
Finocchiaro G, Kobayashi Y, Magavern E, Zhou JQ, Ashley E, Sinagra G, Schnittger I, Knowles JW, Fearon WF, Haddad F, Tremmel JA. Prevalence and Prognostic Role of Right Ventricular Involvement in Stress-Induced Cardiomyopathy. J Cardiac Fail. 2015;21:419–25.
• Stiermaier T, Lange T, Chiribiri A, Möller C, Graf T, Raaz U, Villa A, Kowallick JT, Lotz J, Hasenfuß G, Thiele H, Schuster A, et al. Right ventricular strain assessment by cardiovascular magnetic resonance myocardial feature tracking allows optimized risk stratification in Takotsubo syndrome. PLoS ONE. 2018;13:1–13. This FT-CMR study on TTS patients demonstrated that longitudinal RV strain outperforms subjective visual assessment of RV involvement. It also correlates with EF and identifies individuals at high risk.
Heggemann F, Hamm K, Brade J, Streitner F, Doesch C, Papavassiliu T, Borggrefe M, Haghi D. Right ventricular function quantification in takotsubo cardiomyopathy using two-dimensional strain echocardiography. PLoS One. 2014;9:1–8.
Schuster A, Hor K, Kowallick J, Beerbaum PKS. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016;9:1–9.
• Backhaus SJ, Stiermaier T, Lange T, Chiribiri A, Uhlig J, Freund A, Kowallick JT, Gertz RJ, Bigalke B, Villa A, Lotz J, Hasenfuß G, et al. Atrial mechanics and their prognostic impact in Takotsubo syndrome: A cardiovascular magnetic resonance imaging study. Eur Heart J Cardiovasc Imaging. 2019;20:1059–69. FT-CMR study evaluating biatrial function in the acute phase of TTS demonstrated that LA function during this phase was associated with long-term mortality. This association was independent of traditional cardiovascular risk factors and LVEF.
Cau R, Bassareo P, Caredda G, Suri JS, Esposito A, Saba L. Atrial Strain by Feature-Tracking Cardiac Magnetic Resonance Imaging in Takotsubo Cardiomyopathy. Features, Feasibility, and Reproducibility. Can Assoc Radiol J. 2022;73:573–80.
Stiermaier T, Reil JC, Sequeira V, Rawish E, Mezger M, Pätz T, Paitazoglou C, Schmidt T, Frerker C, Steendijk P, Reil GH, Eitel I. Hemodynamic Assessment in Takotsubo Syndrome. J Am Coll Cardiol. 2023;81:1979–91.
Santoro F, Guastafierro F, Zimotti T, Mallardi A, Leopizzi A, Cannone M, Di Biase M, Brunetti ND. Neutrophil/lymphocyte ratio predicts in-hospital complications in Takotsubo syndrome. Results from a prospective multi-center registry. Clin Cardiol. 2020;43:1294–300.
Yalta K, Yalta T. Physically triggered takotsubo cardiomyopathy has a worse prognosis : Potential roles of systemic in fl ammation and coronary slow fl ow phenomenon. Int J Cardiol. 2017;242:31–2.
Kim M, Gorouhi F, Ramirez S, Granick JL, Byrne BA, Soulika AM, Simon SI, Isseroff RR. Catecholamine Stress Alters Neutrophil Trafficking and Impairs Wound Healing by b 2 -Adrenergic Receptor – Mediated Upregulation of IL-6. J Investig Dermatol. 2014;134:809–17.
Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, Mcguire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–5.
Ciutac AM, Dawson D. Trends in Cardiovascular Medicine The role of inflammation in stress cardiomyopathy. Trends Cardiovasc Med. 2021;31:225–30.
Li Y, Ge S, Peng Y, Chen X. Inflammation and cardiac dysfunction during sepsis, muscular dystrophy, and myocarditis. Burns Trauma. 2013;1:109–21.
Surikow SY, Raman B, Licari J, Singh K, Nguyen TH, Horowitz JD. Evidence of nitrosative stress within hearts of patients dying of Tako-tsubo cardiomyopathy. Int J Cardiol. 2015;189:112–4.
Arcari L, Cimino S, De Luca L, Francone M, Galea N, Reali M, Carbone I, Iacoboni C, Agati L. Impact of heart rate on myocardial salvage in timely reperfused patients with ST-segment elevation myocardial infarction: New insights from cardiovascular magnetic resonance. PLoS ONE. 2015;10:1–12.
Aquaro GD, Ghebru Habtemicael Y, Camastra G, Monti L, Dellegrottaglie S, Moro C, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, et al. Prognostic Value of Repeating Cardiac Magnetic Resonance in Patients With Acute Myocarditis. J Am Coll Cardiol. 2019;74:2439–48.
Todiere G, Pisciella L, Barison A, Del Franco A, Zachara E, Piaggi P, Re F, Pingitore A, Emdin M, Lombardi M, Aquaro GD. Abnormal T2-STIR magnetic resonance in hypertrophic cardiomyopathy: A marker of advanced disease and electrical myocardial instability. PLoS ONE. 2014;9.
Kotecha T, Martinez-Naharro A, Treibel TA, Francis R, Nordin S, Abdel-Gadir A, Knight DS, Zumbo G, Rosmini S, Maestrini V, Bulluck H, Rakhit RD, et al. Myocardial Edema and Prognosis in Amyloidosis. J Am Coll Cardiol. 2018;71:2919–31.
Arcari L, Engel J, Freiwald T, Zhou H, Zainal H, Gawor M, Buettner S, Geiger H, Hauser I, Nagel E, Puntmann VO. Cardiac biomarkers in chronic kidney disease are independently associated with myocardial edema and diffuse fibrosis by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23:1–14.
Arcari L, Cimino S, Filomena D, Monosilio S, Luongo F, Mancone M, Galea N, Francone M, Viviana Maestrini LA. Peak white blood cell count, infarct size and myocardial salvage in patients with reperfused ST-elevation myocardial infarction: a cardiac magnetic resonance study. J Cardiovasc Med. 2021;22:228–30.
Hang W, Chen C, Seubert JM, Wang DW. Fulminant myocarditis: a comprehensive review from etiology to treatments and outcomes. Signal Transduct Target Ther. 2020;5:1–15.
•• Scally C, Abbas H, Ahearn T, Srinivasan J, Mezincescu A, Rudd A, Spath N, Yucel-Finn A, Yuecel R, Oldroyd K, Dospinescu C, Horgan G, et al. Myocardial and systemic inflammation in acute stress-induced (takotsubo) cardiomyopathy. Circulation. 2019;139:1581–92. This multiparametric USPIO-CMR study demonstrated a significant infiltration of activated and phagocytic M1 type macrophages within the myocardial tissue of all TTS patients compared to a matched control group. Additionally, TTS patients showed signs of ongoing low-grade inflammation in the chronic phase.
Cau R, Bassareo P, Cademartiri F, Cadeddu C, Balestrieri A, Mannelli L, Suri JS, Saba L. Epicardial fat volume assessed with cardiac magnetic resonance imaging in patients with Takotsubo cardiomyopathy. Eur J Radiol. 2023;160:110706.
Singh T, Joshi S, Kersahw LE, Baker AH, Dawson DK, Dweck MR, Semple SI, Newby DE. Manganese-enhanced magnetic resonance imaging in Takotsubo syndrome. Eur Heart J. 2022;43:1823–35.
Sörensson P, Ekenbäck C, Lundin M, Agewall S, Bacsovics Brolin E, Caidahl K, Cederlund K, Collste O, Daniel M, Jensen J, Y-Hassan S, Henareh L, et al. Early Comprehensive Cardiovascular Magnetic Resonance Imaging in Patients With Myocardial Infarction With Nonobstructive Coronary Arteries. JACC Cardiovasc Imaging. 2021;14:1774–83.
Gaikwad N, Butler T, Maxwell R, Shaw E, Strugnell WE, Chan J, Figtree G, Hamilton-Craig C. Late gadolinium enhancement does occur in Tako-tsubo cardiomyopathy — A quantitative cardiac magnetic resonance and speckle tracking strain study. IJC Heart Vasc. 2016;12:68–74.
Naruse Y, Sato A, Kasahara K, Makino K, Sano M, Takeuchi Y, Nagasaka S, Wakabayashi Y, Katoh H, Satoh H, Hayashi H, Aonuma K. The clinical impact of late gadolinium enhancement in Takotsubo cardiomyopathy: Serial analysis of cardiovascular magnetic resonance images. J Cardiovasc Magn Reson. 2011;13:67.
Gunasekara MY, Mezincescu AMDD. An Update on Cardiac Magnetic Resonance Imaging in Takotsubo Cardiomyopathy. Curr Cardiovasc Imaging Rep. 2020;13:17.
Dabir D, Luetkens J, Kuetting DLR, Feisst A, Isaak A, Schild HH, Thomas D. Cardiac magnetic resonance including parametric mapping in acute Takotsubo syndrome: Preliminary findings. Eur J Radiol. 2019;113:217–24.
Aikawa Y, Noguchi T, Morita Y, Tateishi E, Kono A, Miura H, Komori Y, Asaumi Y, Fukuda T, Yasuda S. Clinical impact of native T1 mapping for detecting myocardial impairment in takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2019;20:1147–55.
Vermes E, Berradja N, Saab I, Genet T, Bertrand P, Pucheux J, Brunereau L. Cardiac magnetic resonance for assessment of cardiac involvement in Takotsubo syndrome: Do we still need contrast administration? Int J Cardiol. 2020;308:93–5.
Arcari L, Cacciotti L, Camastra G, Ciolina F, Danti M, Sbarbati S, Ansalone G. Cardiac magnetic resonance in Takotsubo syndrome: welcome to mapping, but long live late gadolinium enhancement. Int J Cardiol. 2020;319:150.
• Arcari L, Camastra G, Ciolina F, Limite LR, Danti M, Ansalone G, Musumeci B, Sclafani M, Sbarbati S, Cacciotti L. Myocardial edema contributes to interstitial expansion and associates with mechanical and electrocardiographic changes in takotsubo syndrome: a Cmr T1 and T2 mapping study. Eur Heart J Cardiovasc Imaging 2023;24:1082-1091. This CMR mapping study showed that increased myocardial water content leads to interstitial expansion in acute TTS, which can be detected even outside areas of abnormal wall motion. The burden and distribution of edema were found to be associated with mechanical and electrocardiographic changes.
Arcari L, Limite LR, Adduci C, Sclafani M, Tini G, Palano F, Cosentino P, Cristiano E, Cacciotti L, Russo D, Rubattu S, Volpe M, et al. Novel Imaging and Genetic Risk Markers in Takotsubo Syndrome. Front Cardiovasc Med. 2021;8:1–11.
Migliore F, Zorzi A, Marra MP, Basso C, Corbetti F, De Lazzari M, Tarantini G, Buja P, Lacognata C, Thiene G, Corrado D, Iliceto S. Myocardial edema underlies dynamic T-wave inversion (Wellens’ ECG pattern) in patients with reversible left ventricular dysfunction. Heart Rhythm. 2011;8:1629–34.
Brunetti ND, D’Arienzo G, Sai R, Pellegrino PL, Ziccardi L, Santoro F, Di Biase M. Delayed ventricular pacing failure and correlations between pacing thresholds, left ventricular ejection fraction, and QTc values in a male with Takotsubo cardiomyopathy. Clin Cardiol. 2018;41:1487–90.
Neil C, Nguyen TH, Kucia A, Crouch B, Sverdlov A, Chirkov Y, Mahadavan G, Selvanayagam J, Dawson D, Beltrame J, Zeitz C, Unger S, et al. Slowly resolving global myocardial inflammation/oedema in Tako-Tsubo cardiomyopathy: Evidence from T2-weighted cardiac MRI. Heart. 2012;98:1278–84.
Scally C, Ahearn T, Rudd A, Neil CJ, Srivanasan J, Jagpal B, Horowitz J, Frenneaux M, Dawson DK. Right ventricular involvement and recovery after acute stress-induced (Tako-tsubo) cardiomyopathy. Am J Cardiol. 2016;117:775–80.
Madhavan M, Borlaug BA, Lerman A, Rihal CS, Prasad A. Stress hormone and circulating biomarker profile of apical ballooning syndrome (Takotsubo cardiomyopathy): Insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart. 2009;95:1436–41.
Nguyen TH, Neil CJ, Sverdlov AL, Mahadavan G, Chirkov YY, Kucia AM, Stansborough J, Beltrame JF, Selvanayagam JB, Zeitz CJ, Struthers AD, Frenneaux MP, et al. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am J Cardiol. 2011;108:1316–21.
Pelliccia F, Parodi G, Greco C, Antoniucci D, Brenner R, Bossone E, Cacciotti L, Capucci A, Citro R, Delmas C, Guerra F, Ionescu CS, Lairez O, Larrauri-Reyes M, Hyung Lee P, Mansencal N, Marazzi G, Mihos CG, Morel O, Nef HM, Nunez Gil IJ, Passaseo I, Pine KJ. Comorbidities frequency in Takotsubo syndrome: an international collaborative systematic review including 1109 patients. Am J Med. 2015;128:654.
Cau R, Pisu F, Porcu M, Cademartiri F, Montisci R, Bassareo P, Muscogiuri G, Amadu A, Sironi S, Esposito A, Suri JS, Saba L. Machine learning approach in diagnosing Takotsubo cardiomyopathy: The role of the combined evaluation of atrial and ventricular strain, and parametric mapping. Int J Cardiol. 2023;373:124–33.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. No funding was received.
Author information
Authors and Affiliations
Contributions
MS wrote the main manuscript draft. LA prepared figures 1-2. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Matteo Sclafani, Giacomo Tini, Beatrice Musumeci, Alessandro Cianca, Viviana Maestrini, Luca Cacciotti and Luca Arcari declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sclafani, M., Tini, G., Musumeci, B. et al. Advanced Cardiovascular Magnetic Resonance Imaging in Takotsubo Syndrome: Update on Feature Tracking and Tissue Mapping. Curr Cardiovasc Imaging Rep 17, 61–71 (2024). https://doi.org/10.1007/s12410-024-09593-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12410-024-09593-9