Abstract
Purpose of Review
It is the aim of this review to demonstrate the relevance of stress echocardiography in the era of fractional flow reserve by establishing the current use of stress echocardiography and fractional flow reserve, underlining their physiological basis and through this demonstrating the clear differences in their application.
Recent Findings
The importance of the microcirculation is only now being understood, no more so than in the fact that abnormalities in the microcirculation, determined by abnormal coronary flow reserve, predict adverse mortality regardless of the normality of the epicardial coronary lesions. Stress echocardiography therefore gives a fuller picture of the overall cardiovascular risk to our patients in its ability to interrogate the epicardial vessels down to the microcirculation, with a number of techniques available to measure coronary flow reserve such as myocardial perfusion stress echocardiography and transthoracic Doppler stress echocardiography of epicardial coronary vessels. Fractional flow reserve can then add further information by determining whether a coronary artery lesion is responsible for myocardial ischaemia.
Summary
In an era of fractional flow reserve affording the resolution of myocardial ischaemia down to the specific lesion, it can be tempting to think that other generally non-invasive techniques no longer have a role in the investigation and management of coronary artery disease. This, however, betrays a lack of understanding of the scope and complexity of coronary artery disease from epicardial vessels down to the microvasculature, the physiological basis of the tests available and therefore what, in fact, is actually being measured. For some, fractional flow reserve is held as a gold standard by which to compare other techniques such as stress echocardiography as correct or incorrect. However, these tests do not measure the same thing, and therefore, they cannot be directly compared. Stress echocardiography gives a fuller picture through its ability to account for the coronary flow reserve, considering the epicardial vessels down to the microvasculature. Fractional flow reserve is far more specific, looking at the effect of the lesion being interrogated. Furthermore, where fractional flow reserve is normal, we now know that knowledge of the coronary flow reserve is critical as it is this that allows us to predict the overall mortality risk of our patient. We therefore require a combination of the two techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress echocardiography (SE) is a non-invasive technique that has been used for many years to determine the presence of myocardial ischaemia as well as the extent. Originally validated against nuclear scintigraphy, it has now become the most used technique for ischaemia testing given ease of access, with no need for radioisotopes and being relatively inexpensive. It is even cost-effective when compared with exercise ECG in evaluation of low to intermediate probability coronary artery disease [1]. Whilst SE gives better spatial resolution as compared with nuclear perfusion imaging, invasive testing through coronary angiography has raised the demand for even more specific determination of the functional significance of individual stenoses identified at angiography. Identification of segmental wall thickening abnormalities through SE gives direction as to the likelihood of lesion significance but is not lesion specific. It is this need that has driven the development of the invasive technique of fractional flow reserve.
The use of fractional flow reserve (FFR) was initially demonstrated by Pijls et al. in the early 1990s [2] as a means of measuring the functional severity of a coronary stenosis. Since then, its use has risen in prominence, particularly post the Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) trial which demonstrated that percutaneous coronary intervention (PCI) guided by FFR in multivessel coronary artery disease led to better outcomes mainly driven by less repeat revascularisation [3]. FFR has since become the gold standard in assessment of the functional significance of a coronary stenosis and regarded by some as the invasive alternative to non-invasive ischaemia testing.
However, it must be remembered that FFR was itself initially validated as a test for ischaemia against non-invasive ischaemia testing techniques, initially exercise ECG then later SE and nuclear perfusion imaging. To then use FFR as a gold standard by which to measure the performance of non-invasive tests in determining the presence or absence of ischaemia creates an illogical circularity. Whilst it could be argued that a newer technique can later be shown to be superior to the techniques it was validated against, there is a further issue in the fact that SE and FFR are not measuring exactly the same thing. It is the aim of this review to highlight the current use of SE and FFR, underlining their physiological basis through which clear differences in their application are manifest. The relevance of SE in the era of FFR can then be established.
Stress Echocardiography and CFR
Since its infancy in the 1970s as an M-Mode method showing promise in its ability to demonstrate reduction in wall thickening secondary to myocardial ischaemia, SE has developed over the last 40 years, becoming a useful diagnostic and prognostic tool. Through enhanced harmonic 2D imaging, digitised recording and more recently the use of contrast, diagnostic images can be obtained in most patients.
The underlying principle of SE pertaining to myocardial ischaemia is its ability to demonstrate wall thickening abnormality, occurring with impairment of the left ventricular systolic function as per the ischaemic cascade model [4]. When there is a reduction in myocardial perfusion leading to ischaemia, there is a disproportionate reduction in perfusion of the subendocardial layer versus the subepicardial layer. The mechanism for this is postulated to be due to the distance of the subendocardium from the epicardial vessels, subendocardial proximity to the high-pressure left ventricular cavity during systole [5,6,7], and flow redistribution away from the subendocardium under low perfusion pressure [8]. Since the majority of myocardial wall thickening occurs in the subendocardial layer, up to 80% [9], a reduction in perfusion of the myocardium will manifest in wall thickening reduction.
It is important to note that whilst an epicardial coronary stenosis will commonly cause a reduction in wall thickening, other factors will affect wall thickening such as endothelial dysfunction, microvascular circulatory remodelling and extrinsic compression of the collapsible elements of the microcirculation [10]. It must therefore be appreciated that assessment of wall thickening on SE goes beyond assessment of epicardial coronary artery disease alone but actually assesses disease anywhere from the larger epicardial conductance vessels to the small resistance vessels of the microvasculature (arterioles and capillaries). SE can therefore be used as a surrogate measure of coronary flow reserve (CFR).
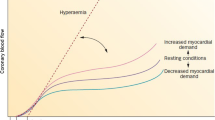
CFR compares myocardial blood flow at maximal metabolic demand with myocardial blood flow at rest, thereby assessing the heart’s ability to respond to increased metabolic demand [11]. To demonstrate this, resting myocardial blood flow is compared with maximal blood flow at hyperaemia using agents such as adenosine and dipyridamole. The ratio of maximal flow during hyperaemia compared with flow at rest gives the coronary flow reserve, with a normal value generally considered as > 2.0 [12] and up to 4.5 in healthy men [13]. However, considering that the CFR is affected by multiple factors other than the condition of the epicardial and microvascular circulation such as heart rate and blood pressure, the CFR can vary with varying baseline conditions.
In the context of an epicardial coronary stenosis, it has been demonstrated in dog models that a stenosis as small as 30% can start to reduce maximal coronary flow thereby reducing the CFR [14]. The mechanism by which this occurs lies in the ability of the resistance vessels to dilate in the face of reduced perfusion pressure to maintain myocardial blood flow at a set rate, so-called coronary autoregulation [15, 16]. However, in doing so, the response to exercise is blunted as there is less capacity to increase myocardial blood flow.
Where there is no significant epicardial coronary disease, the CFR can still be reduced by a number of mechanisms, either by reducing the maximal blood flow or by increasing the basal blood flow. Physiologically increased metabolic demand such as anaemia or sepsis will increase the basal myocardial blood flow leaving less capacity for increase. On the other hand, conditions such as small vessel disease or left ventricular hypertrophy or simply tachycardia, which reduces diastolic perfusion time, will reduce maximal blood flow and therefore CFR [13].
It can be seen, therefore, that the inability to increase myocardial blood flow under stress can occur outside of an epicardial coronary stenosis and lead to ischaemia which will in turn cause a subendocardial wall thickening abnormality detected on SE.
Fractional Flow Reserve
The concept of fractional flow reserve (FFR) was born out of work in dog models of femoral and carotid stenoses investigating pressure changes across a stenosis at maximal hyperaemia compared with resting conditions [17]. The idea of comparing maximal flow across a stenosis to that if there were no stenosis later translated into work by Pijls et al. who validated fractional flow reserve conceptually [2]. Based on the assumption that there was a linear relationship between coronary pressure and flow under conditions of maximal dilatation within the coronary tree, Doppler velocity measures across a stenosis were used as a marker for myocardial blood flow. The changes in these measures correlated very well with the changes in pressure across a stenosis as a marker for myocardial blood flow change. The change in flow due to a coronary stenosis could therefore be determined by measurement of the change in pressure and expressed as the ratio of maximum flow in the presence of stenosis to expected maximum flow in the same distribution without a stenosis, the fractional flow reserve. Mean arterial pressure, Pa, and pressure distal to the stenosis, Pd, are measured and the ratio given Pd/Pa. Maximal flow is achieved through maximal vasodilatation, typically with the use of adenosine.
Once validated as a concept, validation in a clinical physiological context came through comparison with positron emission tomography (PET) of coronary stenoses in humans. Relative flow reserve (RFR) is the non-invasive correlate of FFR and compares maximal flow across a stenosis with that of a normally perfused area, by way of ratio, during a state of maximal vasodilatation (often using adenosine). FFR pressure measurements correlated well with RFR derived from PET [18].
Establishing the clinical utility of FFR in the context of ischaemia was then necessary. This was done through many studies to determine values of FFR that correlated with inducible ischaemia. Originally compared against exercise tests, an FFR value of 0.66 was suggested to best predict an abnormal exercise test [19] with a publication later that year suggesting an FFR ≤ 0.74 was associated with an ischaemia-causing stenosis [20]. Further comparison of FFR with bicycle exercise test, thallium scintigraphy, dobutamine stress echocardiography and quantitative coronary arteriography was made and gave a value of FFR < 0.75 as associated with inducible ischaemia [21]. Multiple other studies have validated the technique of FFR against non-invasive stress techniques including dobutamine stress echocardiography [22, 23] and myocardial perfusion scanning [24,25,26].
Today FFR has become an important tool in the management of patients with coronary artery disease. This is not surprising given its ability to determine the functionally significant ischaemic lesions at a cutoff FFR of < 0.75 and also the ability to risk stratify lesions with an FFR of ≥ 0.75 to low risk that can be deferred with an annual risk of < 1% of cardiac death or myocardial infarction [27].
The use of FFR in everyday coronary angiography was further established in the guidance through the landmark FAME trial [3] which looked at 1005 patients with multivessel coronary artery disease. Patients were randomised to angiography-guided PCI or FFR-guided PCI. A cutoff FFR of 0.8 was used to determine whether to treat a lesion with a drug-eluting stent in the FFR-guided group. Patients with FFR-guided PCI were found to have a significantly reduced event rate at 1 year mainly driven by reduced repeat revascularisation. The FFR-guided strategy led to a lower use of stents (about a third less) with a better outcome. FFR was therefore a superior way of determining significant lesions compared with visual assessment at angiography.
With the ability of FFR to determine the functional significance of coronary lesions, there has been a temptation in some quarters to abandon non-invasive stress tests in favour of the ‘invasive gold standard’ of FFR. However, there is a misconception that FFR and non-invasive tests are measuring the same substrate and FFR does it better. Taking SE as the focus of this review, we have shown above that SE measures the CFR which takes into account the microvasculature. Whilst it will determine the presence or absence of inducible ischaemia for an epicardial coronary stenosis, it is argued that FFR will do this with greater spatial resolution in that it determines the culprit vessel and not simply a territory. This is true but is not a justification for simply replacing SE with FFR. Dispensing with SE would mean dispensing with additional prognostic information gained by SE’s ability to investigate the microvasculature.
CFR-FFR Discordance and the Microcirculation
The importance of the microcirculation is becoming more apparent with its investigation. Nothing highlights this fact more than the emerging evidence for poorer prognosis in patients with microvascular disease. Britten et al. considered the effect of a reduced CFR in a population of patients who were angiographically normal or had minimal disease, i.e. no significant epicardial coronary stenoses [28]. Doppler catheters were used to assess blood flow in the target vessel and either papaverine or adenosine was used to achieve hyperaemia. They showed that the patients with the lowest tertile of coronary flow reserve had a significantly higher incidence of cardiovascular event up to 10 years compared with those within the middle or highest tertile. Cardiovascular events included cardiovascular death, myocardial infarction, stroke revascularisation and unstable angina.
The Women’s Ischaemia Syndrome Evaluation (WISE) [29] set out to investigate whether coronary microvascular dysfunction in women with ischaemic symptoms referred for angiography led to adverse outcomes. A Doppler-tipped guidewire was used to measure intracoronary velocity and adenosine for hyperaemia. Analysis of the data from women without obstructive coronary artery disease showed significantly more major adverse outcomes in those with low CFR.
More specific consideration of effects on mortality due to microvascular dysfunction has been made in recent years. Hoef et al. measured the coronary flow velocity reserve (CFVR) through intracoronary Doppler flow velocity in patients who had just received primary percutaneous coronary intervention for an ST segment elevation myocardial infarction (STEMI). Doppler flow velocity measures were taken from the culprit vessel immediately post intervention as well as a normal reference vessel (defined as having < 30% diameter stenosis). Over a 10-year follow-up period, there was a significant difference in cardiac mortality when comparing high with low reference vessel CFVR. Those with a high CFVR (≥ 2.1) had a 5% mortality compared with 31% mortality in the low (< 2.1) CFVR group [30]. Study of CFVR in stable coronary artery disease of at least 1 intermediate coronary lesion yielded similar results. Twelve-year estimates for cardiac mortality in patients with normal reference vessel CFVR (> 2.7) were 7.7% compared with 31.6% in patients with abnormal reference vessel CFVR (≤ 2.7) [31].
Thus, it can be seen that low CFR predicts cardiac mortality in the long term. Therefore, SE, which accounts for the CFR, would be expected to also predict cardiac mortality, which it does. Patients who have a negative exercise echocardiogram have been shown to have a mortality of 1% per year at 6 years’ follow-up of over 5000 patients [32]. Pharmacological stress echocardiography with either dobutamine or dipyridamole has been investigated in over 7000 patients and a negative test shown to predict a very low risk of death with a < 1% mortality a year [33].
With a wealth of data behind the prognostic value of a negative stress echocardiogram, the same cannot be said for FFR. Whilst the 5-year follow-up data of the DEFER trial [27] showed that an FFR ≥ 0.75 predicts a low risk for patients, with an annual risk of < 1% of cardiac death or myocardial infarction, there has been conflicting evidence. In stable coronary artery disease, the use of a drug-eluting stent for a stenotic vessel with FFR < 0.8 does not confer a mortality benefit over medical therapy alone. It does, however, lead to significantly less urgent revascularisation. There is also the consideration that an FFR > 0.8 still predicts a cardiovascular event rate of 2.6% a year [34•].
Added to this, a well-documented discordance in the results from FFR and CFR has been the cause of much speculation. Table 1 gives an example of this discordance. This discordance in the results for abnormality ranges from 30 to 40% [35]. The question often raised by the difference is ‘Which result is wrong?’ With the physiological variability inherent in the measurement of CFR, it is often assumed that this is the errant result. However, one must be mindful that FFR is also subject to variability of conditions, particularly of the fact that a reduction in trans-stenotic flow could lead to an incorrectly higher FFR reading.
CFR-FFR discordance has been studied in the literature and has led to some interesting conclusions. Work using fluid dynamic models for the FFR-CFR interaction concluded on a linear relationship with the balance between focal stenosis, diffuse coronary artery disease and small vessel or microvascular disease determining the slope [36]. Discordance would then occur with the extremes of diffuse or microvascular disease versus focal disease. Thus, neither FFR nor CFR is wrong but varies differently dependent on the nature of the underlying disease.
A particularly interesting finding is that in the face of a normal FFR and abnormal CFR, a patient has a worse prognosis than a patient with an abnormal FFR and normal CFR [35]. This has also been shown through FFR versus SE, where SE is a surrogate for CFR. Wall thickening abnormality is associated with cardiovascular events whereas FFR is not [34•]. Figure 1 demonstrates the ability of SE to determine the risk of cardiovascular events (death or non-fatal myocardial infarction) where FFR is negative. Where FFR is truly normal and CFR abnormal, the abnormality in the coronary circulation is predominantly from the microcirculation [35]. Thus, the microcirculation has a larger role to play than has been appreciated to now and the understanding of this will be critical to affecting mortality in the context of myocardial ischaemia and beyond in the future.
Kaplan-Meier survival curve demonstrating freedom from CV events in SE-positive FFR-negative versus SE-negative FFR-negative patients. Reproduced from S. Gurunathan et al., “Diagnostic Concordance and Clinical Outcomes in Patients Undergoing Fractional Flow Reserve and Stress Echocardiography for the Assessment of Coronary Stenosis of Intermediate Severity,” J. Am. Soc. Echocardiogr., vol. 31, no. 2, pp. 180–186, 2018
Doppler SE and Myocardial Contrast Echocardiography
SE has thus far been discussed in the context of its ability to measure wall thickening. There have, however, been major advances in the field of echocardiography with newer more technically demanding techniques offering new ways to interrogate the coronary circulation, particularly the microcirculation.
Interrogation of coronary arteries began with Doppler investigation of coronary artery bypass grafts for patency [37, 38] as well as visualisation of the left main coronary artery in the 1970s [39]. Coronary blood flow to the mid portion and distal left anterior descending artery (LAD) was measured with relative success in the 1980s using two-dimensional echocardiography with Doppler [40, 41]. It did not then take long to realise the potential of transthoracic pulsed Doppler measures of velocity in the LAD to be used in the calculation of CFR. Hozumi et al. showed the value of measuring CFVR in diseased and non-diseased LADs with CFVR of < 2.0 using mean diastolic flow velocities having a sensitivity of 92% and specificity of 86% for significant coronary stenosis [42]. They then went on to validate the technique against the established Doppler guidewire determination of CFVR showing accurate reflection of invasive measurements by the non-invasive transthoracic Doppler echocardiography (TTDE) technique [43]. The same has been demonstrated using TTDE in the posterior descending artery portion of the right coronary artery (RCA) [44, 45].
As a technique, alignment with the coronary vessel is critical, as it relies on Doppler readings, making the left circumflex the most challenging artery to interrogate. However, it is possible to interrogate the left circumflex artery but with less accuracy than the LAD and RCA [46]. That being said, the use of intravenous contrast has made the measurement of CFR possible in the LAD in most patients in experienced hands with a feasibility of 97% in an unselected group of patients with median BMI above 30 previously demonstrated [47].
With the fact that SE assessment of wall thickening abnormalities acts as a surrogate measure of CFR, one can ask of the added benefit of TTDE, particularly as it is a more technically demanding technique. This was addressed by Cortigiani et al. when they looked at pulsed Doppler echocardiography in the LAD during dipyridamole stress echocardiography. They investigated 4313 patients in a prospective, multicentre observational study looking at all-cause mortality as an endpoint. They showed that a positive SE by wall motion criteria and impaired CFR by pulsed Doppler conferred a particularly bad prognosis with annual mortality > 10%. However, if the SE was normal with a normal CFR, annual mortality was < 1%. The most interesting finding was that of added prognostic value of CFR assessment of the LAD over SE wall motion analysis. If a patient demonstrated no wall motion abnormality on SE but had a CFR ≤ 2, they had an increased risk of mortality likely reflecting prognostically significant microvascular disease not manifesting in discernible wall motion abnormality on SE [48].
A second technique that has advanced the scope of SE in recent times is myocardial perfusion stress echocardiography (MPSE). This technique has the advantage over regular SE assessment for wall thickening in that it assesses perfusion and so detects ischaemia at an earlier point in the ischaemic cascade. The physiological basis for MPSE lies in the fact that most of the myocardial blood volume lies in the microcirculation. Intravenous microbubble contrast infusion fills this microcirculation which is visible on 2D transthoracic echocardiography at low or very low mechanical index settings. At steady state, this reflects the myocardial blood volume by intensity of contrast signal. With destruction of the microbubbles with high mechanical index ultrasound waves (MI > 0.8), they will replenish the microcirculation and the rate at which this occurs reflects the myocardial blood velocity. Therefore, the technique allows assessment of myocardial blood flow through myocardial blood volume and velocity (the product of which gives myocardial blood flow). An example of a study is given in Fig. 2.
An example of a stress-induced perfusion defect in the left circumflex coronary artery territory (arrows). Note that end-systolic replenishment within the basal to mid inferolateral segments in the apical long-axis window is normal under resting conditions but delayed (arrows) during dobutamine stress imaging. Reproduced from T. R. Porter et al., “Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update.,” J. Am. Soc. Echocardiogr., vol. 31, no. 3, pp. 241–274, Mar. 2018
Acquisition of perfusion images is made at rest and stress with a perfusion abnormality identified by a combined assessment of reduced signal intensity to a territory (myocardial blood volume) and an increase in time taken to replenish the vascular territory (myocardial blood velocity). Validation of MPSE to accurately measure myocardial blood flow has been made using positron emission tomography (PET) [49]. Furthermore, its value clinically has been validated against single-photon emission computed tomography (SPECT) for detection of coronary artery disease [50].
The question of what MPSE adds over and above conventional SE is likely to lie in its ability to more directly assess the microcirculation through measuring myocardial blood flow as well as the fact that assessment of perfusion defects occurs earlier in the ischaemic cascade and therefore should increase the sensitivity in detecting myocardial ischaemia. The enhanced sensitivity of MPSE for the detection of flow-limiting CAD has been shown in several studies, and it was also shown to translate into improved prognosis [51]. This has resulted in the guidelines and recommendations for routine use of MPSE in clinical practice [51, 52•]. The ability of the technique to reproducibly assess CFR has also been validated against invasive Doppler wire measurement, and so in expert hands, MPSE can be seen to be a robust technique offering information over and above conventional SE [53]. Whether this translates into further significant prognostication remains to be seen.
Conclusion
Stress echocardiography is a widely used technique available to most medical facilities with no need for highly specialised equipment. It has found its place in clinical practice in determination of the presence and significance of coronary artery disease. Its high negative predictive value is a particular strength in ruling out significant coronary artery disease. The use of FFR has brought us back to the physiologic underpinnings of the pathology we see. However, it appears that we have stopped at FFR and not delved further into the physiological basis of the pathology of the entire coronary vasculature. Some have used FFR as a gold standard with which to discount the results of SE, which do not agree with FFR, as incorrect. However, it is far more logical that where two tests measure different things, the difference in results will reflect this. And so it is the ability of SE to measure abnormalities in the microcirculation, where FFR cannot, that leads to discordance in results.
It is only when we truly understand what we are measuring that we can know what to do with the results. Is there a place for SE in the era of FFR, most certainly yes. Its ability to measure CFR directly through Doppler interrogation of coronary arteries or MPSE or through the surrogate measure of wall thickening affords us precious prognostic information that FFR simply cannot. FFRs strength is in its spatial resolution, able to resolve the artery responsible for the ischaemia that has been detected symptomatically or by non-invasive test. However, where FFR is normal, the normality or not of the CFR is critical as it is this that allows us to know the overall mortality risk of our patient. It can therefore be seen that we need both FFR and SE for the fullest picture with neither excluding the need for the other.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Gurunathan S, Zacharias K, Akhtar M, Ahmed A, Mehta V, Karogiannis N, et al. Cost-effectiveness of a management strategy based on exercise echocardiography versus exercise electrocardiography in patients presenting with suspected angina during long term follow up: a randomized study. Int J Cardiol. 2018;259:1–7.
Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354–67.
Tonino PAL, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24.
Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987;59(7):23C–30C.
van den Wijngaard JPHM, et al. Model prediction of subendocardial perfusion of the coronary circulation in the presence of an epicardial coronary artery stenosis. Med Biol Eng Comput. 2008;46(5):421–32.
DOMENECH RJ, GOICH J. Effect of heart rate on regional coronary blood flow. Cardiovasc Res. 1976;10(2):224–31.
Hoffman JI, Spaan JA. Pressure-flow relations in coronary circulation. Physiol Rev. 1990;70(2):331–90.
Algranati D, Kassab GS, Lanir Y. Why is the subendocardium more vulnerable to ischemia? A new paradigm. Am J Physiol Heart Circ Physiol. 2011;300(3):H1090–100.
Gurunathan S, Senior R. Stress echocardiography in stable coronary artery disease. Curr Cardiol Rep. 2017;19(12):121.
Van De Hoef TP, et al. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat Rev Cardiol. 2013;10(8):439–52.
MOSHER P, ROSS J, MCFATE PA, SHAW RF. Control of coronary blood flow by an autoregulatory mechanism. Circ Res. 1964;14:250–9.
Kern MJ, Bach RG, Mechem CJ, Caracciolo EA, Aguirre FV, Miller LW, et al. Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol. 1996;28(5):1154–60.
Meuwissen M, et al. Role of fractional and coronary flow reserve in clinical decision making in intermediate coronary lesions. Interv Cardiol. 2009;1(2):237–55.
Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33(1):87–94.
Chilian WM. Coronary microcirculation in health and disease summary of an NHLBI workshop. Circulation. 1997;95(2):522.
Camici PG, Crea F. Coronary microvascular dysfunction - reply. N Engl J Med. 2007;356(22):2325.
Young DF, Cholvin NR, Kirkeeide RL, Roth AC. Hemodynamics of arterial stenoses at elevated flow rates. Circ Res. 1977;41(1):99–107.
De Bruyne B, et al. Coronary flow reserve calculated from pressure measurements in humans. Circulation. 1994;89:1013–22.
De Bruyne B, Bartunek J, Sys SU, Heyndrickx GR. Relation between myocardial fractional flow reserve calculated from coronary pressure measurements and exercise-induced myocardial ischemia. Circulation. 1995;92(1):39–46.
Pijls NH, van Gelder B, van der Voort P, Peels K, Bracke FA, Bonnier HJ, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92(11):3183–93.
Pijls NHJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703–8.
Bartunek J, et al. Dobutamine-induced wall motion abnormalities: correlations with myocardial fractional flow reserve and quantitative coronary angiography. J Am Coll Cardiol. 1996;27(6):1429–36.
Jiménez-Navarro M, Alonso-Briales JH, Hernández García MJ, Rodríguez Bailón I, Gómez-Doblas JJ, de Teresa Galván E. Measurement of fractional flow reserve to assess moderately severe coronary lesions: correlation with dobutamine stress echocardiography. J Interv Cardiol. 2001;14(5):499–504.
Caymaz O, Fak AS, Tezcan H, Inanir SS, Toprak A, Tokay S, et al. Correlation of myocardial fractional flow reserve with thallium-201 SPECT imaging in intermediate-severity coronary artery lesions. J Invasive Cardiol. 2000;12(7):345–50.
Chamuleau SAJ, et al. Fractional flow reserve, absolute and relative coronary blood flow velocity reserve in relation to the results of technetium-99m sestamibi single-photon emission computed tomography in patients with two-vessel coronary artery disease. J Am Coll Cardiol. 2001;37(5):1316–22.
Yanagisawa H, Chikamori T, Tanaka N, Hatano T, Morishima T, Hida S, et al. Correlation between thallium-201 myocardial perfusion defects and the functional severity of coronary artery stenosis as assessed by pressure-derived myocardial fractional flow reserve. Circ J. 2002;66(12):1105–9.
Pijls NHJ, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49(21):2105–11.
Britten MB, Zeiher AM, Schächinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15(5):259–64.
Pepine CJ, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–32.
Van De Hoef TP, et al. Impact of coronary microvascular function on long-term cardiac mortality in patients with acute ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2013;6(3):207–15.
Van De Hoef TP, et al. Impaired coronary autoregulation is associated with long-term fatal events in patients with stable coronary artery disease. Circ Cardiovasc Interv. 2013;6(4):329–35.
Marwick TH, Case C, Vasey C, Allen S, Short L, Thomas JD. Prediction of mortality by exercise echocardiography: a strategy for combination with the duke treadmill score. Circulation. 2001;103(21):2566–71.
Sicari R, Pasanisi E, Venneri L, Landi P, Cortigiani L, Picano E. Stress echo results predict mortality: a large-scale multicenter prospective international study. J Am Coll Cardiol. 2003;41(4):589–95.
• Gurunathan S, et al. Diagnostic concordance and clinical outcomes in patients undergoing fractional flow reserve and stress echocardiography for the assessment of coronary stenosis of intermediate severity. J Am Soc Echocardiogr. 2018;31(2):180–6 This study highlights the fact that wall thickening assessment by stress echocardiography better predicts cardiovascular events than FFR measurement.
Van De Hoef TP, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ. Cardiovasc. Interv. 2014;7(3):301–11.
Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012;5(2):193–202.
Gould KL, Mozersky DJ, Hokanson DE, Baker DW, Kennedy JW, Sumner DS, et al. A noninvasive technic for determining patency of saphenous vein coronary bypass grafts. Circulation. 1972;46(3):595–600.
Pisko-Dubienski ZA, Baird RJ, Wilson DR. Noninvasive assessment of aorta-coronary saphenous vein bypass graft patency using directional Doppler. Circulation. 1975;52(2 Suppl):I188–97.
Weyman AE, Feigenbaum H, Dillon JC, Johnston KW, Eggleton RC. Noninvasive visualization of the left main coronary artery by cross-sectional echocardiography. Circulation. 1976;54(2):169–74.
Fusejima K. Noninvasive measurement of coronary artery blood flow using combined two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 1987;10(5):1024–31.
Ross JJ, Mintz GS, Chandrasekaran K. Transthoracic two-dimensional high frequency (7.5 MHz) ultrasonic visualization of the distal left anterior descending coronary artery. J Am Coll Cardiol. 1990;15(2):373–7.
Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, et al. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation. 1998;97(16):1557–62.
Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, et al. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32(5):1251–9.
Ueno Y, Nakamura Y, Takashima H, Kinoshita M, Soma A. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the right coronary artery by transthoracic Doppler echocardiography: comparison with intracoronary Doppler guidewire. J Am Soc Echocardiogr. 2002;15(10 Pt 1):1074–9.
Ueno Y, Nakamura Y, Kinoshita M, Fujita T, Sakamoto T, Okamura H. Noninvasive assessment of significant right coronary artery stenosis based on coronary flow velocity reserve in the right coronary artery by transthoracic Doppler echocardiography. Echocardiography. 2003;20(6):495–501.
Murata E, Hozumi T, Matsumura Y, Fujimoto K, Sugioka K, Takemoto Y, et al. Coronary flow velocity reserve measurement in three major coronary arteries using transthoracic Doppler echocardiography. Echocardiography. 2006;23(4):279–86.
Olsen RH, et al. Coronary flow velocity reserve by echocardiography: feasibility, reproducibility and agreement with PET in overweight and obese patients with stable and revascularized coronary artery disease. Cardiovasc Ultrasound. 2016;14(1):22.
Cortigiani L, Rigo F, … S. G.-J., and undefined. Coronary flow reserve during dipyridamole stress echocardiography predicts mortality. 2012. imaging.onlinejacc.org.
Vogel R, et al. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. J Am Coll Cardiol. 2005;45(5):754–62.
Peltier M, Vancraeynest D, Pasquet A, Ay T, Roelants V, D’hondt A, et al. Assessment of the physiologic significance of coronary disease with dipyridamole real-time myocardial contrast echocardiography. Comparison with technetium-99m sestamibi single-photon emission computed tomography and quantitative coronary angiography. J Am Coll Cardiol. 2004;43(2):257–64.
Senior R, et al. Clinical practice of contrast echocardiography: recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur Heart J - Cardiovasc Imaging. 2017;18(11):1205–1205af.
• Porter TR, et al. Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography guidelines update. J Am Soc Echocardiogr. 2018;31(3):241–74 A thorough appraisal of the utility and importance of ultrasonic enhancing agents in current practice of echocardiography.
Wei K, Ragosta M, Thorpe J, Coggins M, Moos S, Kaul S. Noninvasive quantification of coronary blood flow reserve in humans using myocardial contrast echocardiography. Circulation. 2001;103(21):2560–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Echocardiography
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bioh, G., Senior, R. Stress Echocardiography in the Era of Fractional Flow Reserve. Curr Cardiovasc Imaging Rep 13, 6 (2020). https://doi.org/10.1007/s12410-020-9528-y
Published:
DOI: https://doi.org/10.1007/s12410-020-9528-y