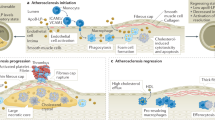
Abstract
Purpose of Review
The purpose of this review is to highlight some of the recent developments (within the last 5 years) in imaging atherosclerotic plaques using nanoparticles, with a focus on technologies that have been applied to in vivo models of disease.
Recent Findings
Structural and cellular components of atherosclerotic plaques are being imaged with greater definition and improved sensitivity. This is a result of both molecular targeting of nanoparticles to disease-relevant biomarkers through the use of nanoparticles of different shapes and sizes and tailoring pharmacokinetic parameters that allow for enhanced pharmacodynamic effects.
Summary
Currently, there are no atherosclerotic plaque imaging techniques clinically validated to predict future clinical events. Considering the rapid pace of new nanomaterial discovery and development, along with the development of multimodality imaging systems, this goal seems within reach. These advancements are “nano” in name only.









Similar content being viewed by others
References
World Health Organization. The top 10 causes of death. http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Acessed 15 Jan 2019.
Vigne J, Thackeray J, Essers J, Makowski M, Varasteh Z, Curaj A, et al. Current and emerging preclinical approaches for imaging-based characterization of atherosclerosis. Mol Imaging Biol. 2018;20(6):869–87. https://doi.org/10.1007/s11307-018-1264-1.
Smith BR, Gambhir SS. Nanomaterials for in vivo imaging. Chem Rev. 2017;117(3):901–86. https://doi.org/10.1021/acs.chemrev.6b00073.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914.
Koenig W. Inflammation revisited: atherosclerosis in the post-CANTOS era. Eur Cardiol. 2017;12(2):89–91. https://doi.org/10.15420/ecr.2017:18:1.
Woodside DG, Tanifum EA, Ghaghada KB, Biediger RJ, Caivano AR, Starosolski ZA, et al. Magnetic resonance imaging of atherosclerotic plaque at clinically relevant field strengths (1T) by targeting the integrin alpha4beta1. Sci Rep. 2018;8(1):3733. https://doi.org/10.1038/s41598-018-21893-x.
Bruckman MA, Jiang K, Simpson EJ, Randolph LN, Luyt LG, Yu X, et al. Dual-modal magnetic resonance and fluorescence imaging of atherosclerotic plaques in vivo using VCAM-1 targeted tobacco mosaic virus. Nano Lett. 2014;14(3):1551–8. https://doi.org/10.1021/nl404816m.
Chen W, Cormode DP, Vengrenyuk Y, Herranz B, Feig JE, Klink A, et al. Collagen-specific peptide conjugated HDL nanoparticles as MRI contrast agent to evaluate compositional changes in atherosclerotic plaque regression. JACC Cardiovasc Imaging. 2013;6(3):373–84. https://doi.org/10.1016/j.jcmg.2012.06.016.
Yu M, Ortega CA, Si K, Molinaro R, Schoen FJ, Leitao RFC, et al. Nanoparticles targeting extra domain B of fibronectin-specific to the atherosclerotic lesion types III, IV, and V-enhance plaque detection and cargo delivery. Theranostics. 2018;8(21):6008–24. https://doi.org/10.7150/thno.24365.
Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M, et al. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci U S A. 2009;106(24):9815–9. https://doi.org/10.1073/pnas.0903369106.
Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15(2):104–16. https://doi.org/10.1038/nri3793.
Nakayama M. Macrophage recognition of crystals and nanoparticles. Front Immunol. 2018;9:103. https://doi.org/10.3389/fimmu.2018.00103.
Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278(5):483–93. https://doi.org/10.1111/joim.12406.
Cybulsky MI, Gimbrone MA Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–91.
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107(10):1255–62. https://doi.org/10.1172/JCI11871.
Patel SS, Thiagarajan R, Willerson JT, Yeh ET. Inhibition of alpha4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation. 1998;97(1):75–81.
Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol. 2009;27:165–97. https://doi.org/10.1146/annurev.immunol.021908.132620.
Libby P, Pasterkamp G, Crea F, Jang IK. Reassessing the mechanisms of acute coronary syndromes. Circ Res. 2019;124(1):150–60. https://doi.org/10.1161/CIRCRESAHA.118.311098.
Reimann C, Brangsch J, Colletini F, Walter T, Hamm B, Botnar RM, et al. Molecular imaging of the extracellular matrix in the context of atherosclerosis. Adv Drug Deliv Rev. 2017;113:49–60. https://doi.org/10.1016/j.addr.2016.09.005.
Sinha A, Shaporev A, Nosoudi N, Lei Y, Vertegel A, Lessner S, et al. Nanoparticle targeting to diseased vasculature for imaging and therapy. Nanomedicine. 2014;10(5):1003–12. https://doi.org/10.1016/j.nano.2014.02.002.
Perez-Medina C, Binderup T, Lobatto ME, Tang J, Calcagno C, Giesen L, et al. In vivo PET imaging of HDL in multiple atherosclerosis models. JACC Cardiovasc Imaging. 2016;9(8):950–61. https://doi.org/10.1016/j.jcmg.2016.01.020.
Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem Int Ed Eng. 2007;46(43):8171–3. https://doi.org/10.1002/anie.200700700.
Kaspar M, Zardi L, Neri D. Fibronectin as target for tumor therapy. Int J Cancer. 2006;118(6):1331–9. https://doi.org/10.1002/ijc.21677.
Matter CM, Schuler PK, Alessi P, Meier P, Ricci R, Zhang D, et al. Molecular imaging of atherosclerotic plaques using a human antibody against the extra-domain B of fibronectin. Circ Res. 2004;95(12):1225–33. https://doi.org/10.1161/01.RES.0000150373.15149.ff.
Dietrich T, Berndorff D, Heinrich T, Hucko T, Stepina E, Hauff P, et al. Targeted ED-B fibronectin SPECT in vivo imaging in experimental atherosclerosis. Q J Nucl Med Mol Imaging. 2015;59(2):228–37.
Kim H, Lee Y, Kang S, Choi M, Lee S, Kim S, et al. Self-assembled nanoparticles comprising aptide-SN38 conjugates for use in targeted cancer therapy. Nanotechnology. 2016;27(48):48LT01. https://doi.org/10.1088/0957-4484/27/48/48LT01.
Starmans LW, van Duijnhoven SM, Rossin R, Aime S, Daemen MJ, Nicolay K, et al. SPECT imaging of fibrin using fibrin-binding peptides. Contrast Media Mol Imaging. 2013;8(3):229–37. https://doi.org/10.1002/cmmi.1521.
Starmans LW, van Duijnhoven SM, Rossin R, Berben M, Aime S, Daemen MJ, et al. Evaluation of 111In-labeled EPep and FibPep as tracers for fibrin SPECT imaging. Mol Pharm. 2013;10(11):4309–21. https://doi.org/10.1021/mp400406x.
Starmans LW, Moonen RP, Aussems-Custers E, Daemen MJ, Strijkers GJ, Nicolay K, et al. Evaluation of iron oxide nanoparticle micelles for magnetic particle imaging (MPI) of thrombosis. PLoS One. 2015;10(3):e0119257. https://doi.org/10.1371/journal.pone.0119257.
Yoo SP, Pineda F, Barrett JC, Poon C, Tirrell M, Chung EJ. Gadolinium-functionalized peptide amphiphile micelles for multimodal imaging of atherosclerotic lesions. ACS Omega. 2016;1(5):996–1003. https://doi.org/10.1021/acsomega.6b00210.
Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci U S A. 2007;104(3):932–6. https://doi.org/10.1073/pnas.0610298104.
Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114(14):1504–11. https://doi.org/10.1161/CIRCULATIONAHA.106.646380.
Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, et al. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2(10):1213–22. https://doi.org/10.1016/j.jcmg.2009.04.016.
McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur Muhlen C, et al. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28(1):77–83. https://doi.org/10.1161/ATVBAHA.107.145466.
Stein-Merlob AF, Hara T, McCarthy JR, Mauskapf A, Hamilton JA, Ntziachristos V, et al. Atheroma susceptible to thrombosis exhibit impaired endothelial permeability in vivo as assessed by nanoparticle-based fluorescence molecular imaging. Circ Cardiovasc Imaging 2017;10(5) doi:https://doi.org/10.1161/CIRCIMAGING.116.005813.
Wang Y, Chen J, Yang B, Qiao H, Gao L, Su T, et al. In vivo MR and fluorescence dual-modality imaging of atherosclerosis characteristics in mice using profilin-1 targeted magnetic nanoparticles. Theranostics. 2016;6(2):272–86. https://doi.org/10.7150/thno.13350.
Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114(4):622–34. https://doi.org/10.1093/cvr/cvy007.
Winkels H, Ehinger E, Ghosheh Y, Wolf D, Ley K. Atherosclerosis in the single-cell era. Curr Opin Lipidol. 2018;29(5):389–96. https://doi.org/10.1097/MOL.0000000000000537.
Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018;122(12):1675–88. https://doi.org/10.1161/CIRCRESAHA.117.312513.
Cochain C, Vafadarnejad E, Arampatzi P, Pelisek J, Winkels H, Ley K, et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122(12):1661–74. https://doi.org/10.1161/CIRCRESAHA.117.312509.
Cole JE, Park I, Ahern DJ, Kassiteridi C, Danso Abeam D, Goddard ME, et al. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res. 2018;114(10):1360–71. https://doi.org/10.1093/cvr/cvy109.
Konopka CJ, Wozniak M, Hedhli J, Ploska A, Schwartz-Duval A, Siekierzycka A, et al. Multimodal imaging of the receptor for advanced glycation end-products with molecularly targeted nanoparticles. Theranostics. 2018;8(18):5012–24. https://doi.org/10.7150/thno.24791.
Majmudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res. 2013;112(5):755–61. https://doi.org/10.1161/CIRCRESAHA.111.300576.
Keliher EJ, Ye YX, Wojtkiewicz GR, Aguirre AD, Tricot B, Senders ML, et al. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat Commun. 2017;8:14064. https://doi.org/10.1038/ncomms14064.
Lee JM, Choudhury RP. Atherosclerosis regression and high-density lipoproteins. Expert Rev Cardiovasc Ther. 2010;8(9):1325–34. https://doi.org/10.1586/erc.10.108.
Brown WV, Brewer HB, Rader DJ, Schaefer EJ. HDL as a treatment target. J Clin Lipidol. 2010;4(1):5–16. https://doi.org/10.1016/j.jacl.2009.12.005.
Karalis I, Jukema JW. HDL mimetics infusion and regression of atherosclerosis: is it still considered a valid therapeutic option? Curr Cardiol Rep. 2018;20(8):66. https://doi.org/10.1007/s11886-018-1004-9.
Mulder WJM, van Leent MMT, Lameijer M, Fisher EA, Fayad ZA, Perez-Medina C. High-density lipoprotein nanobiologics for precision medicine. Acc Chem Res. 2018;51(1):127–37. https://doi.org/10.1021/acs.accounts.7b00339.
Zheng KH, van der Valk FM, Smits LP, Sandberg M, Dasseux JL, Baron R, et al. HDL mimetic CER-001 targets atherosclerotic plaques in patients. Atherosclerosis. 2016;251:381–8. https://doi.org/10.1016/j.atherosclerosis.2016.05.038.
Tang J, Lobatto ME, Hassing L, van der Staay S, van Rijs SM, Calcagno C, et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv. 2015;1(3):e1400223. https://doi.org/10.1126/sciadv.1400223.
Seijkens TTP, van Tiel CM, Kusters PJH, Atzler D, Soehnlein O, Zarzycka B, et al. Targeting CD40-induced TRAF6 signaling in macrophages reduces atherosclerosis. J Am Coll Cardiol. 2018;71(5):527–42. https://doi.org/10.1016/j.jacc.2017.11.055.
Wen S, Liu DF, Cui Y, Harris SS, Chen YC, Li KC, et al. In vivo MRI detection of carotid atherosclerotic lesions and kidney inflammation in ApoE-deficient mice by using LOX-1 targeted iron nanoparticles. Nanomedicine. 2014;10(3):639–49. https://doi.org/10.1016/j.nano.2013.09.009.
Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25(5):419–29. https://doi.org/10.1007/s10557-011-6341-5.
Nie S, Zhang J, Martinez-Zaguilan R, Sennoune S, Hossen MN, Lichtenstein AH, et al. Detection of atherosclerotic lesions and intimal macrophages using CD36-targeted nanovesicles. J Control Release. 2015;220(Pt A):61–70. https://doi.org/10.1016/j.jconrel.2015.10.004.
Tarin C, Carril M, Martin-Ventura JL, Markuerkiaga I, Padro D, Llamas-Granda P, et al. Targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI. Sci Rep. 2015;5:17135. https://doi.org/10.1038/srep17135.
Beldman TJ, Senders ML, Alaarg A, Perez-Medina C, Tang J, Zhao Y, et al. Hyaluronan nanoparticles selectively target plaque-associated macrophages and improve plaque stability in atherosclerosis. ACS Nano. 2017;11(6):5785–99. https://doi.org/10.1021/acsnano.7b01385.
Luehmann HP, Detering L, Fors BP, Pressly ED, Woodard PK, Randolph GJ, et al. PET/CT imaging of chemokine receptors in inflammatory atherosclerosis using targeted nanoparticles. J Nucl Med. 2016;57(7):1124–9. https://doi.org/10.2967/jnumed.115.166751.
Bagalkot V, Deiuliis JA, Rajagopalan S, Maiseyeu A. "Eat me" imaging and therapy. Adv Drug Deliv Rev. 2016;99(Pt A:2–11. https://doi.org/10.1016/j.addr.2016.01.009.
Poh S, Putt KS, Low PS. Folate-targeted dendrimers selectively accumulate at sites of inflammation in mouse models of ulcerative colitis and atherosclerosis. Biomacromolecules. 2017;18(10):3082–8. https://doi.org/10.1021/acs.biomac.7b00728.
Moreno-Layseca P, Icha J, Hamidi H, Ivaska J. Integrin trafficking in cells and tissues. Nat Cell Biol. 2019;21:122–32. https://doi.org/10.1038/s41556-018-0223-z.
Chen CL, Siow TY, Chou CH, Lin CH, Lin MH, Chen YC, et al. Targeted superparamagnetic iron oxide nanoparticles for in vivo magnetic resonance imaging of T-cells in rheumatoid arthritis. Mol Imaging Biol. 2017;19(2):233–44. https://doi.org/10.1007/s11307-016-1001-6.
Capolla S, Garrovo C, Zorzet S, Lorenzon A, Rampazzo E, Spretz R, et al. Targeted tumor imaging of anti-CD20-polymeric nanoparticles developed for the diagnosis of B-cell malignancies. Int J Nanomedicine. 2015;10:4099–109. https://doi.org/10.2147/IJN.S78995.
Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106(23):2894–900.
Doring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–43. https://doi.org/10.1161/CIRCRESAHA.116.309692.
Pertiwi KR, van der Wal AC, Pabittei DR, Mackaaij C, van Leeuwen MB, Li X, et al. Neutrophil extracellular traps participate in all different types of thrombotic and haemorrhagic complications of coronary atherosclerosis. Thromb Haemost. 2018;118(6):1078–87. https://doi.org/10.1055/s-0038-1641749.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. https://doi.org/10.1126/science.1092385.
Pellico J, Lechuga-Vieco AV, Almarza E, Hidalgo A, Mesa-Nunez C, Fernandez-Barahona I, et al. In vivo imaging of lung inflammation with neutrophil-specific (68)Ga nano-radiotracer. Sci Rep. 2017;7(1):13242. https://doi.org/10.1038/s41598-017-12829-y.
Acknowledgments
The author would like to thank Peter Vanderslice, PhD, for critical review of the manuscript, and Heather Leibrecht, MS, ELS, of The Texas Heart Institute for editorial assistance in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Woodside has a patent pending, PCT/US18/29991—Targeting Nanoparticles, and he is a co-founder of and investor in 7Hills Pharma, LLC, a startup biotechnology company developing novel immunotherapies for cancer.
Human and Animal Rights and Informed Consent
The article reviews work previously published by the author that involved animals. There are no unpublished animal studies included in this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Molecular Imaging
Rights and permissions
About this article
Cite this article
Woodside, D.G. Nanoparticle Imaging of Vascular Inflammation and Remodeling in Atherosclerotic Disease. Curr Cardiovasc Imaging Rep 12, 28 (2019). https://doi.org/10.1007/s12410-019-9501-9
Published:
DOI: https://doi.org/10.1007/s12410-019-9501-9