Abstract
Background
Dormant coronary collaterals are highly prevalent and clinically beneficial in cases of coronary occlusion. However, the magnitude of myocardial perfusion provided by immediate coronary collateral recruitment during acute occlusion is unknown. We aimed to quantify collateral myocardial perfusion during balloon occlusion in patients with coronary artery disease (CAD).
Methods
Patients without angiographically visible collaterals undergoing elective percutaneous transluminal coronary angioplasty (PTCA) to a single epicardial vessel underwent two scans with 99mTc-sestamibi myocardial perfusion single-photon emission computed tomography (SPECT). All subjects underwent at least three minutes of angiographically verified complete balloon occlusion, at which time an intravenous injection of the radiotracer was administered, followed by SPECT imaging. A second radiotracer injection followed by SPECT imaging was performed 24 h after PTCA.
Results
The study included 22 patients (median [interquartile range] age 68 [54-72] years. The perfusion defect extent was 19 [11-38] % of the LV, and the collateral perfusion at rest was 64 [58-67]% of normal.
Conclusion
This is the first study to describe the magnitude of short-term changes in coronary microvascular collateral perfusion in patients with CAD. On average, despite coronary occlusion and an absence of angiographically visible collateral vessels, collaterals provided more than half of the normal perfusion.
Similar content being viewed by others
Background
The coronary collateral circulation is a preformed network of anastomotic connections between epicardial arteries acting as a “natural bypass” mechanism.1,2 The presence of robust coronary collateral circulation is known to be associated with improved survival and left ventricular function in the setting of ST-elevation myocardial infarction (STEMI).3
Whilst the prevalence of collaterals varies between species, approximately 25% of patients have angiographically visible robust collaterals at the time of ST elevation myocardial infarction.4 Short-term changes in collateral flow in humans have been described angiographically by Rentrop et al.1 In that study, patients with stable angina and single-vessel disease were studied by contralateral coronary artery contrast injection during balloon inflation. Most patients had no collateral filling at baseline, while all but one patient showed improved collateral filling upon balloon occlusion. This suggests a high prevalence of dormant collaterals, but does not provide reliable information on magnitude of the resultant perfusion.
Importantly, collateral vessels smaller than approximately 100 μm are not visibly detectable by invasive angiography.5 However, relative myocardial perfusion can be quantified by 99mTc-sestamibi single-photon emission computed tomography (SPECT) regardless of vessel size. 99mTc-sestamibi uptake is proportional to myocardial perfusion,6 and it exhibits minimal redistribution after initial uptake.7 While the contribution of collateral flow has been thoroughly described in canine models using microsphere techniques,8,9,10,11,12,13 there is no quantitative data on short-term changes in collateral perfusion in man. Of note, infarct biology cannot be readily translated from canine models to man,14 and an understanding of human basic coronary physiology is essential to cardiovascular medicine. Therefore, the aim of the study was to quantify collateral myocardial perfusion during coronary balloon occlusion in patients with CAD using 99mTc-sestamibi SPECT.
Methods
Study design
To quantify the amount of collateral perfusion in CAD patients, two myocardial perfusion single-photon emission computed tomography (SPECT) scans were performed in all patients. One of them after isotope injection during complete coronary occlusion (Occlusion study), and one at rest (Control study). The difference in myocardial perfusion between the Occlusion study and the Control study reflects the difference in perfusion between a state of immediate and complete coronary artery balloon occlusion and a non-occluded state, respectively. Patients with angiographically visible collaterals were excluded, and the difference in perfusion can thus be considered to be an effect of recruitment of dormant collaterals, recruited immediately upon coronary occlusion.
The current study was a retrospective substudy of a previously published larger cohort.15 In that study, 42 patients with obstructive, single-vessel coronary artery disease underwent elective prolonged balloon inflation during percutaneous transluminal coronary angioplasty (PTCA), and two myocardial perfusion SPECT scans between September 1995 and April 1996 with radioactive tracer injections during coronary occlusion and at rest the subsequent day.15 A flow-chart of the study protocol is presented in Figure 1.
Due to either technical problems, unsuccessful injections, or prior coronary artery bypass surgery to the balloon-occluded artery, 7 patients were excluded from the original study. For the purpose of this study, patients with angiographically visible collaterals, prior infarction, or coronary artery bypass surgery (not only to the balloon-occluded artery) were further excluded (n = 13).
The study was undertaken at Charleston Area Medical Center, Charleston, West Virginia, USA, and was approved by the local Investigational Review Board. Ethical permission was granted based on prior favorable clinical, pilot experience with the methodology, and that subjects all could provide informed consent for this elective procedure, which was obtained for all participants.
SPECT imaging
For each patient, when the angioplasty balloon was angiographically verified as being fully inflated during PTCA, with absence of anterograde blood flow, 1100 MBq (30 mCi) 99mTc-sestamibi was injected intravenously. The intracoronary balloon inflation lasted for at least three minutes in all patients, which allowed for adequate clearance of 99mTc-sestamibi from the bloodstream.16 Immediately following PTCA, the interventional cardiologist performing the procedure recorded the location of balloon occlusion and the duration of balloon inflation. Also, at the conclusion of the procedure, the interventional cardiologist noted whether angiographically visible collateral circulation was considered to be present upon review of the angiographic images of all coronary vessels. Within three hours following angioplasty, the patient was brought to the nuclear medicine laboratory to acquire SPECT imaging data (Occlusion study). On the day following PTCA, a second intravenous injection with 1100 MBq of 99mTc-sestamibi was administered intravenously, followed by SPECT imaging within three hours (Control study). Each patient in the study was clinically stable throughout both processes.
SPECT imaging was performed using a single-head gamma camera (Elscint, Haifa, Israel) with a 140 keV (± 20%) energy window. Images were acquired using a low energy high-resolution collimator in a 64 × 64 matrix, 6.9 mm pixel size, using 30 projections (25 s/projection) over 180° from 45° right anterior oblique to 45° left posterior oblique. A filtered back projection with a Butterworth filter (order 5, cut-off 0.25 cycles/pixel) was used to reconstruct axial slices. The same gamma camera and image acquisition procedures were used for the Occlusion and Control studies.
Image Analysis
Short-axis images were reconstructed using the Cedars-Emory quantitative analysis software (CEqual), and were used to build a volume-weighted bull’s eye plots.17The quantification of collateral perfusion was performed by comparing the Occlusion study to the Control study for each patient. The Control study was first corrected by taking the decay of 99mTc into account (24 h, Tc99m decay constant λ = 0.1151 h−1). Following this process, the Occlusion study was scaled so that its average value, in the region above 90% of its maximum, was made equal to the average value of the control study in the same region of the heart. This normalization was necessary to achieve uniformity for the Control and Occlusion studies in regions not impacted by inflation-induced hypoperfusion.
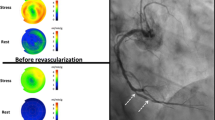
The amount of residual perfusion downstream of the balloon-occluded artery, presumably due to coronary collateral flow, was quantified within the region of hypoperfusion in the following manner. First, the ratio of perfusion between the occlusion study and the control study was calculated. Secondly, the extent of the perfusion defect was identified in an extent map, and the region where the ratio of Occlusion-to-Control counts was less than 75% of tracer uptake was delineated, as previously validated.15 Residual collateral perfusion, i.e. the perfusion within the perfusion defect in % of the normal perfusion within that same region, was calculated as Occlusion counts divided by Control counts (%). Representative cases are illustrated in Figure 2.
Visualization of collateral perfusion to the left ventricular myocardium during elective percutaneous transluminal coronary angioplasty (PTCA) in patients with coronary artery disease. Polar plots show left ventricular (LV) myocardial perfusion by 99mTc-sestamibi SPECT. A Balloon-occluded right coronary artery. B Balloon-occluded left circumflex coronary artery. Images show the myocardial perfusion during balloon occlusion (upper left images within A and B respectively, “Occlusion”), and 24 h later at rest (upper right images within A and respectively, “Control”). First, the occlusion study was normalized to the control study (see text). A new polar plot was then constructed (lower left images, “Occlusion/Control”) by calculating the ratio between the Occlusion study and the Control study. Based on the new polar plot, a perfusion defect (lower right images, “Perfusion defect”) was defined as the region where the Occlusion/Control ratio was less than 75% 4. Residual myocardial perfusion, i.e. collateral perfusion, within that defect was quantified as Occlusion counts divided by Control counts, expressed in percent
Statistical Analysis
Non-normally distributed data are presented as median [interquartile range]. Differences between groups were tested using the Wilcoxon test. Linear correlations were described using Spearman’s rank correlation coefficient ρ. A two-sided P-value less than 0.05 was considered statistically significant. All statistical analysis was performed using the software R (version 4.0.4, R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
The study included 22 patients (age 68 [54-72] years, 10 (45%) female). The degree of diameter stenosis of treated vessels ranged from 60 to 99%, with successful PTCA performed with a mean balloon occlusion time of 5 min (range 3-7 min), resulting in ≤ 20% residual stenosis in all cases. Ten patients underwent stent implantation as part of the procedure. The vessels undergoing PTCA were 6 (27%) in the left anterior descending artery (LAD) [4 proximal], 7 (32%) in the left circumflex coronary artery (LCx) [5 proximal] and 9 (41%) in the right coronary artery (RCA) [4 proximal].
For the entire cohort, the median extent of the perfusion defect was 19 [11-38] % of the left ventricle. The extent of perfusion defects was larger in patients with LAD occlusion (47 [42-50] %), compared to RCA or LCx occlusion (147,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 %), P < 0.001. The residual collateral perfusion was 64 [58-67] % [Figure 3], 51 [48-54]% in LAD occlusion, and 65 [63-69]% in RCA or LCx occlusion (P < 0.001). Collateral perfusion was negatively correlated with perfusion defect size (ρ = − 0.82, P < 0.001), but did not differ by sex (males 62 [54-66] vs females 65 [61-68] %, P = 0.34) and was not correlated to age (ρ = − 0.18, P = 0.42).
Discussion
The main finding of the study was that immediate recruitment of dormant collaterals after balloon occlusion of an epicardial coronary artery results in substantial myocardial perfusion, averaging 64% of normal perfusion. This is the first study to describe the magnitude of short-term changes in collateral perfusion in human subjects with CAD. A high prevalence of short-term changes in collateral flow was described angiographically by Rentrop, et al., using contralateral contrast injections during balloon inflation, with improvement in angiographic collateral filling grade in all but one patient.1 However, angiographic visualization of coronary vessels does not necessarily equal myocardial perfusion.18 We add to the existing knowledge by providing quantification of the myocardial perfusion resulting from coronary collateral recruitment. Our findings are in agreement with previous findings that collateral flow, in some cases, can be enough to prevent short-term ischemia and anginal symptoms.19
Although the observation of significant collateral perfusion upon acute coronary occlusion does not provide immediate clinical implications, an understanding of the underlying physiological processes related to acute ischemic heart disease provide an essential foundation for future clinical innovation. This study provides a basis for further research in this area, e.g. regarding how collateral perfusion affects clinical outcomes, what factors affect its magnitude, and how/if different therapies affect the magnitude of collateral perfusion.
Collateral coronary Perfusion in Humans
The magnitude of collateral perfusion in our study was greater than previously described. Collateral perfusion has been evaluated in patients with successfully reperfused STEMI, and found to provide incremental information on final infarct size, beyond myocardium at risk, time to reperfusion, and infarct location.20 In that study, 89 patients were injected with 99mTc-sestamibi prior to revascularization by PTCA or thrombolysis. Collateral myocardial perfusion was estimated by comparing the counts within the perfusion defect to the maximal value within the same short-axis slice, and was found to be approximately 27%, which is less than in our study. Of note, in contrast to our methodology, the authors did not evaluate the collateral perfusion in reference to the normal perfusion within the affected myocardial region, but rather it was compared to remote myocardium in the same slice. Furthermore, that study reported the magnitude of collateral perfusion in a clinical cohort before emergent revascularization, after on average 5 h of acute coronary occlusion and ongoing myocardial infarction. In contrast to the results of that study, the current is the first study to report the magnitude of perfusion in relation to normal perfusion that can be accomplished by collateral vessel recruitment in an immediate (3-5 min) response to coronary occlusion without the cofounding pathophysiology of ongoing acute myocardial infarction which involves a significantly longer duration of coronary occlusion. Our data stems from previous research in which the correlation between ST-segment changes and myocardial ischemia during coronary occlusion was evaluated.16 Although the severity of ischemia was described in that paper, the current study included patients without visible coronary collaterals only, thus it provides specific insights into recruitment of dormant collateral coronary vessels.
Collateral Perfusion in Animal Models
By comparison, Reimer, et al., have reported from experimental studies in the dog, that collateral flow decreases over time in coronary occlusion, especially in subendocardial regions.8,12
The magnitude of collateral perfusion in humans, as shown in our study, is higher than in animal models.8,9,10 Reimer, et al., found variable but consistently lower collateral flow in dogs with a proximally occluded LCx, ranging from 12 to 31% in subendocardial vs. subepicardial layers of the myocardium, in reference to normally perfused myocardium,8 and even values as low as 6% have been reported.10 By comparison, the current study found more than twofold greater collateral perfusion in patients with CAD, likely due to a combination of biological differences between species, age, and the presence of CAD.
Technical Considerations
The current study showed a negative correlation between perfusion defect size and the magnitude of collateral perfusion. In other words, smaller perfusion defects had greater collateral perfusion than larger perfusion defects. Phantom studies have shown that residual activity in perfusion defects is influenced not only by the collateral flow to that region, but also by pixel size, collimator resolution, type of orbit, defect size, and location. With the SPECT technology used in this study, a significant overestimation can be expected in defect sizes < 10%, but the effect is of less importance with larger defect sizes.21 By excluding those with an extent < 10% the median residual collateral perfusion was still substantial (62%). The difference in the extent of perfusion defects between LAD and RCA or LCx occlusions likely contributes to the observed differences in residual perfusion between different ischemic locations. Taken together, these results suggest that although technical aspects can have influenced the results, there may be a limit to the distance over which collaterals can effectively perfuse into the core of a perfusion defect.
The Clinical Importance of Collaterals
Having robust coronary collateral circulation as visualized by invasive angiography has been associated with clinical benefits, since robust collaterals allow oxygenated blood to reach the jeopardized myocardium.2,3,22 Patients with robust collaterals presenting with STEMI have a lower mortality in both the short and long term, and higher left ventricular ejection fraction.3 Furthermore, patients with robust collaterals are more likely to have successful percutaneous coronary intervention to treat chronic total occlusion.22 Future studies on coronary collateral circulation could incorporate quantification of the myocardial perfusion contributed by collaterals using myocardial perfusion imaging methods.
Limitations
The data used in this study stem from a study performed during the years 1995-1996. Since then, myocardial perfusion imaging has undergone substantial technical, radiopharmaceutical, and computational advances.23,24,25,26,27,28 However, any technical limitation present would have equally affected both the Occlusion and Control study, and not necessarily had a sizable impact on the magnitude of collateral coronary perfusion detected in the current study. Nonetheless, future studies evaluating immediate coronary collateral recruitment, and seeking to replicate the current findings, would be of value, using modern, state-of-the-art imaging techniques.
The access to data from complete epicardial coronary artery balloon occlusion and simultaneous radioactive tracer injection with control imaging in human subjects is unique. If the results are cautiously interpreted, significant knowledge can be gained from these records, despite apparent limitations.
Of note, concluding that the residual perfusion within the perfusion defect can be explained by immediate recruitment of dormant collaterals relies on the hypothesis that the resting perfusion obtained the day after PTCA would equal the perfusion conditions prior to PTCA. Possibly, small collaterals that were not angiographically detectable may have been present. Also, regional overlap of coronary circulation in the border regions of the myocardium may have affected the uptake within the defined perfusion defect, falsely attributing all residual uptake to collateral perfusion. However, this would likely have had a minor impact on the larger perfusion defects, which still showed substantial residual perfusion.
Uptake of 99mTc-sestamibi following balloon deflation could potentially falsely increase the estimation of collateral perfusion. However, < 5% of the activity remains in the blood stream at 5 min after intravenous injection.29 Since mean balloon inflation time in our study was 5 min, this likely had minimal impact on our results.
Furthermore, contralateral vessel angiography was not performed during balloon inflation in this study. Thus, it is only known that collateral vessels could not be visualized prior to balloon occlusion, but it is not known if collateral vessels could be angiographically visualized during balloon occlusion, which otherwise would be the case for example in the assessment of chronic total coronary occlusion. Regardless, the angiographic visualization of collateral vessels was performed under clinical conditions, and the current results highlight that angiographic visualization under clinical conditions does not visualize a sizable portion of perfusion that can reach myocardium subtended by a given artery should it become occluded. Also, SPECT images were not corrected for attenuation, which may affect accuracy, albeit not substantially,30 and with minimal impact since the same attenuating properties would be at play during both the Occlusion and Control study.
Some degree of collateral perfusion was present in all patients in this study, but the sample size is small and the study lacks information on detailed clinical characteristics to conclude which factors affect the magnitude of collateral perfusion.
Conclusions
This is the first study to quantify myocardial perfusion after immediate collateral recruitment following coronary occlusion in patients with CAD. On average, collaterals provided more than half of normal myocardial perfusion in patients with CAD. Future research on diagnosis and therapy in CAD could consider quantification of collateral perfusion beyond angiographic visualization.
New knowledge gained
Short-term changes in collateral flow in humans have been described angiographically but the magnitude of myocardial perfusion provided by immediate coronary collateral recruitment during acute occlusion is unknown. In patients with coronary artery disease who underwent complete coronary balloon occlusion collaterals provided more than half of normal myocardial perfusion.
Abbreviations
- CAD:
-
Coronary artery disease
- LAD:
-
Left anterior descending coronary artery
- LCx:
-
Left circumflex coronary artery
- LV:
-
Left ventricle
- PTCA:
-
Percutaneous transluminal coronary angioplasty
- RCA:
-
Right coronary artery
- SPECT:
-
Single-photon emission computed tomograpy
- STEMI:
-
ST-elevation myocardial infarction
- 99mTc:
-
Technetium-99 m
References
Rentrop KP, Cohen M, Blanke H, et al. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5:587‐92.
Rentrop KP, Feit F, Sherman W, et al. Serial angiographic assessment of coronary artery obstruction and collateral flow in acute myocardial infarction. Report from the second Mount Sinai-New York University Reperfusion Trial. Circulation. 1989;80:1166‐75.
Allahwala UK, Nour D, Alsanjari O, et al. Prognostic implications of the rapid recruitment of coronary collaterals during ST elevation myocardial infarction (STEMI): a meta-analysis of over 14,000 patients. J Thromb Thrombolysis 2021;51:1005‐16.
Allahwala UK, Weaver JC, Nelson GI, et al. Effect of recruitment of acute coronary collaterals on in-hospital mortality and on left ventricular function in patients presenting with ST elevation myocardial infarction. Am J Cardiol 2020;125:1455‐60.
Gensini GG,Bruto da Costa BC. The coronary collateral circulation in living man. Am J Cardiol. 1969;24:393-400.
Sinusas AJ, Trautman KA, Bergin JD, et al. Quantification of area at risk during coronary occlusion and degree of myocardial salvage after reperfusion with technetium-99m methoxyisobutyl isonitrile. Circulation 1990;82:1424‐37.
Haronian HL, Remetz MS, Sinusas AJ, et al. Myocardial risk area defined by technetium-99m sestamibi imaging during percutaneous transluminal coronary angioplasty: comparison with coronary angiography. J Am Coll Cardiol 1993;22:1033‐43.
Reimer KA, Jennings RB, Cobb FR, et al. Animal models for protecting ischemic myocardium: results of the NHLBI Cooperative Study. Comparison of unconscious and conscious dog models. Circ Res. 1985;56:651‐65.
Murdock RH Jr, Chu A, Grubb M, et al. Effects of reestablishing blood flow on extent of myocardial infarction in conscious dogs. Am J Physiol 1985;249:H783‐91.
Ugander M, Bagi PS, Oki AJ, et al. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging 2012;5:596‐603.
Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633‐44.
Reimer KA, Lowe JE, Rasmussen MM, et al. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 1977;56:786‐94.
Jennings RB, Ganote CE, Reimer KA. Ischemic tissue injury. Am J Pathol 1975;81:179‐98.
Hedström E, Engblom H, Frogner F, et al. Infarct evolution in man studied in patients with first-time coronary occlusion in comparison to different species - implications for assessment of myocardial salvage. J Cardiovasc Magn Reson 2009;11:38.
Persson E, Palmer J, Pettersson J, et al. Quantification of myocardial hypoperfusion with 99m Tc-sestamibi in patients undergoing prolonged coronary artery balloon occlusion. Nucl Med Commun 2002;23:219‐28.
Persson E, Pettersson J, Pahlm O, et al. Comparison of ST-segment deviation to scintigraphically quantified myocardial ischemia during acute coronary occlusion induced by percutaneous transluminal coronary angioplasty. Am J Cardiol 2006;97:295‐300.
Garcia EV, Cooke CD, Van Train KF, et al. Technical aspects of myocardial SPECT imaging with technetium-99m sestamibi. Am J Cardiol 1990;66:23e‐31e.
Knuuti J, Ballo H, Juarez-Orozco LE, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J 2018;39:3322‐30.
Wustmann K, Zbinden S, Windecker S, et al. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation 2003;107:2213‐20.
Christian TF, Gibbons RJ, Clements IP, et al. Estimates of myocardium at risk and collateral flow in acute myocardial infarction using electrocardiographic indexes with comparison to radionuclide and angiographic measures. J Am Coll Cardiol 1995;26:388‐93.
O’Connor MK, Gibbons RJ, Juni JE, et al. Quantitative myocardial SPECT for infarct sizing: feasibility of a multicenter trial evaluated using a cardiac phantom. J Nucl Med 1995;36:1130‐6.
Allahwala UK, Nour D, Bhatia K, et al. Prognostic impact of collaterals in patients with a coronary chronic total occlusion: A meta-analysis of over 3,000 patients. Catheter Cardiovasc Interv 2021;97:E771‐7.
Mannarino T, D’Antonio A, Assante R, et al. Regional myocardial perfusion imaging in predicting vessel-related outcome: interplay between the perfusion results and angiographic findings. Eur J Nucl Med Mol Imaging 2022;50:160‐7.
Cantoni V, Green R, Ricciardi C, et al. A machine learning-based approach to directly compare the diagnostic accuracy of myocardial perfusion imaging by conventional and cadmium-zinc telluride SPECT. J Nucl Cardiol 2022;29:46‐55.
Gaudieri V, Mannarino T, Zampella E, et al. Prognostic value of coronary vascular dysfunction assessed by rubidium-82 PET/CT imaging in patients with resistant hypertension without overt coronary artery disease. Eur J Nucl Med Mol Imaging 2021;48:3162‐71.
Oddstig J, Hedeer F, Jögi J, et al. Reduced administered activity, reduced acquisition time, and preserved image quality for the new CZT camera. J Nucl Cardiol 2013;20:38‐44.
Mouden M, Timmer JR, Ottervanger JP, et al. Impact of a new ultrafast CZT SPECT camera for myocardial perfusion imaging: fewer equivocal results and lower radiation dose. Eur J Nucl Med Mol Imaging 2012;39:1048‐55.
Herzog BA, Buechel RR, Katz R, et al. Nuclear myocardial perfusion imaging with a cadmium-zinc-telluride detector technique: optimized protocol for scan time reduction. J Nucl Med 2010;51:46‐51.
Boschi A, Uccelli L, Marvelli L, et al. Technetium-99m Radiopharmaceuticals for Ideal Myocardial Perfusion Imaging: Lost and Found Opportunities. Molecules. 2022;27.
Slomka PJ, Fish MB, Lorenzo S, et al. Simplified normal limits and automated quantitative assessment for attenuation-corrected myocardial perfusion SPECT. J Nucl Cardiol 2006;13:642‐51.
Acknowledgements
The authors thank medical physicist Dr. Enid Eslick, PhD, for valuable discussion and input regarding nuclear medicine physics-related aspects of the methodology.
Funding
Open access funding provided by Karolinska Institute. TL: The Swedish Heart-Lung Foundation (Grant No. 20200553), the Swedish Cardiac Society, the Royal Swedish Academy of Sciences (Grant No. LM2019-0013), Women and Health Foundation, Region Kronoberg (Grant No. 8301), The Swedish Heart and Lung Association (Grant No. LKH1387), Swedish Association of Clinical Physiology, and the Scandinavian Society of Clinical Physiology & Nuclear Medicine. MU: New South Wales Health, Heart Research Australia, and the University of Sydney.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
All authors have no relevant conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reid, B.J., Lindow, T., Warren, S. et al. Immediate recruitment of dormant coronary collaterals can provide more than half of normal resting perfusion during coronary occlusion in patients with coronary artery disease. J. Nucl. Cardiol. 30, 2338–2345 (2023). https://doi.org/10.1007/s12350-023-03271-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-023-03271-x