Abstract
Introduction
This systematic review aims to verify the efficacy of acarbose monotherapy in treating obese or overweight patients without diabetes.
Methods
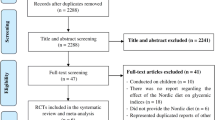
In the study, we conducted a systematic search of the Pub-Med, EMBASE, Cochrane and Science Citation Index Expanded databases in search of clinical trials on acarbose treatment, overweight and obesity. The crucial inclusion criteria were as follows: (1) patients were diagnosed as overweight or obese (BMI ≥ 25 kg/m2); (2) randomized controlled trials (RCTs); (3) patients had undergone acarbose monotherapy or placebo control; (4) acarbose treatment had been carried out for at least 3 months. Exclusion criteria were as follows: (1) patients diagnosed with diabetes mellitus (DM); (2) patients had received a weight loss medication or surgery in the past 3 months; (3) papers not published in English; (4) repeated research results of the same experiment or repeated published documents.
Results
A total of 7 studies involving 132 in the acarbose group and 137 in placebo group, 269 subjects in total, were included in this meta-analysis. From the selected seven papers, we extracted the following clinical parameters: systolic blood pressure (SBP), diastolic blood pressure (DBP), body weight (BW), body mass index (BMI), triglyceride (TG), total cholesterol (TC), low density lipoprotein (LDL), high density cholesterol (HDL) and fasting plasma glucose (FPG). An important finding of our research is that TG was the only significantly reduced parameter in the acarbose group. Weight mean difference (WMD) was − 0.21 (95% CI − 0.33, − 0.09) mmol/l between acarbose (P = 0.0006) and placebo patients. Reduction of BMI was also greater for acarbose than placebo subjects, although the discrepancy was not statistically significant (P = 0.56). Moreover, no hypoglycemia occurred in either the acarbose group or placebo group. A few subjects experienced gastrointestinal reactions, but these were mild and improved over time. Acarbose has no obvious influence on other metabolic indexes.
Conclusion
Acarbose monotherapy is beneficial in reducing TG levels in obese or overweight patients and will not result in hypoglycemia during medication. The side effects of acarbose are mild.










Similar content being viewed by others
Change history
19 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12325-021-01653-6
References
Rachmani R, Bar-Dayan Y, Ronen Z, et al. The effect of acarbose on insulin resistance in obese hypertensive subjects with normal glucose tolerance: a randomized controlled study[J]. Diabetes Obes Metab. 2004;6(1):63–8.
Bayraktar F, Hamulu F, Ozgen AG, et al. Acarbose treatment in obesity: a controlled study[J]. Eat Weight Disord. 1998;3(1):46–9.
Hauner H, Petzinna D, Sommerauer B, et al. Effect of acarbose on weight maintenance after dietary weight loss in obese subjects[J]. Diabetes Obes Metab. 2001;3(6):423–7.
Laube H, Linn T, Heyen P. The effect of acarbose on insulin sensitivity and proinsulin in overweight subjects with IGT[J]. Exp Clin Endocrinol Diabetes. 1998;106(03):231–3.
Penna IAA, Canella PRB, Vieira CS, et al. Cardiovascular risk factors are reduced with a low dose of acarbose in obese patients with polycystic ovary syndrome[J]. Fertil Steril 2007;88(2):519–22.
Chiasson JL. The effect of acarbose on insulin sensitivity in subjects with IGT[J]. Diabet Med. 1996;13(2):23–4.
Malaguarnera M, Giugno I, Ruello P, et al. Treatment of familial hypertriglyceridaemia with acarbose[J]. Diabetes Obes Metab. 1999;2(1):33–8.
Yu Chung Chooi. The epidemiology of obesity[J]. Metab Clin Exp. 2019;92:6–10.
Reilly JJ. Health effects of overweight and obesity in 195 countries[J]. N Engl J Med. 2017;377(15):1496.
Smetanina N, Albaviciute E, Babinska V, Karinauskiene L, Albertsson-Wikland K, Petrauskiene A, Verkauskiene R. Prevalence of overweight/obesity in relation to dietary habits and lifestyle among 7–17 years old children and adolescents in Lithuania. BMC Public Health. 2015;1(15):1001.
Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Eng J Med. 2013;369(2):145–54.
Guang Y, Chunlin Li. Application of α -glucosidase inhibitor in diabetic patients [J]. Chin J Drug Appl Monit. 2007;01:16–20.
Hanefeld M, Pistrosch F, Koehler C, et al. Conversion of IGT to type 2 diabetes mellitus is associated with incident cases of hypertension[J]. J Hypertens. 2012;30(7):1440–3.
Noushin K, Alireza S. Evaluation of the effects of acarbose on weight and metabolic, inflammatory, and cardiovascular markers in patients with obesity and overweight[J]. Int J Prev Med. 2020;11:140.
Nakhaee A, Sanjari M. Evaluation of effect of acarbose consumption on weight losing in non-diabetic overweight or obese patients in Kerman[J]. J Res Med Sci. 2013;18(5):391–4.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Li N, Zhong L, Wang C, et al. Cemented versus uncemented hemi-arthroplasty for femoral neck fractures in elderly patients: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99:e19039.
Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome[J]. Endocrine. 2006;29:109–17 (PMID: 16622298).
Kissane NA, Pratt JSA. Medical and surgical treatment of obesity[J]. Best Practice Res Clin Anaesthesiol. 2011;25(1):11–25.
Martin KA, Mani MV, Mani A. New targets to treat obesity and the metabolic syndrome[J]. Eur J Pharmacol. 2015;763:S0014299915004574.
Ying C, Qingsen L. Progress in non-surgical treatment of adult obesity [J]. J Air Force General Hospital. 2010;26(04):220–4.
Xiangli C, Wen T, Min S. Observation of 60 cases of obesity with igt treated by acarbose intervention[J]. Chin J Misdiagnos. 2003;3(11):1675–6.
Ramirez I. Does reducing the rate of ef®ciency of digestion reduce food intake[J]. Am J Physiol. 1992;263:R852–6.
Siraj ES. Is there a role for metformin or acarbose as a weight-loss agent in the absence of diabetes[J]? Cleve Clin J Med. 2003;70:702–4.
Halpern A, Mancini MC, Suplicy H, Zanella MT, Repetto G, Gross J, et al. Latin American trial of orlistat for weight loss and improvement in glycaemic profile in obese diabetic patients[J]. Diabetes Obes Metab. 2003;5:180–8.
Hokanson JE, Austin MA. Plasma triglyceride level as risk factor for cardiovascular disease independent of high density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies[J]. J Cardiovasc Risk. 1996;3:213–9.
Inoue I, Shinoda Y, Nakano T, et al. Acarbose ameliorates atherogenecity of low-density lipoprotein in patients with IGT[J]. Metab-Clin Exp. 2006;55(7):946–52.
Penna IAA. Acarbose in obese patients with polycystic ovarian syndrome: a double-blind, randomized, placebo-controlled study[J]. Hum Reprod. 2005;20(9):2396–401.
Malaguarnera M, Giugno I, Ruello P, et al. Treatment of familial hypertriglyceridaemia with acarbose[J]. Diabetes Obes Metab. 2000;2(1):33–8.
Pan CY, Gao Y, Chen JW, et al. Efficacy of acarbose in Chinese subjects with IGT[J]. Diabe Res Clin Pract. 2003;61(3):183–90.
Liqing C, Airong Y. Systematic evaluation of acarbose in the treatment of IGT[J]. Chin Pharmacy. 2015;26(18):2509–12.
Carrascosa JM, Molero JC, Fermín Y, et al. Effects of chronic treatment with acarbose on glucose and lipid metabolism in obese diabetic Wistar rats[J]. Diabetes Obes Metab. 2001;3(4):240–8.
Pérez C, Fernández-Agulló T, De Solís AJ, et al. Effects of chronic acarbose treatment on adipocyte insulin responsiveness, serum levels of leptin and adiponectin and hypothalamic NPY expression in obese diabetic Wistar rats[J]. Clin Exp Pharmacol Physiol. 2008;35(3):256–61.
Ogawa S, Takeuchi K, Ito S. Acarbose lowers serum triglyceride and postprandial chylomicron levels in type 2 diabetes[J]. Diabetes Obes Metab. 2004;6(5):384–90.
Malaguarnera M, Giugno I, Ruello P, et al. Acarbose is an effective adjunct to dietary therapy in the treatment of hypertriglyceridaemias[J]. Br J Clin Pharmacol. 1999;48(4):605–9.
Kahn SE, Prigeon RL, Mcculloch DK, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects[J]. Diabetes. 1993;42(11):1663–72.
Maogao X, Ping C. Effect of adjuvant therapy with Bifidobacterium triple viable capsule on β cell function and insulin resistance in newly diagnosed type 2 diabetes patients[J]. Chin J Microecol. 2017;29(4):454–6.
Bischoff H. The mechanism of alpha-glucosidase inhibition in the management of diabetes[J]. Clin Invest Med. 1995;18:303–11.
Khalili N, Safavipour A. Evaluation of the effects of acarbose on weight and metabolic, inflammatory, and cardiovascular markers in patients with obesity and overweight[J]. Int J Prev Med. 2020;11:140.
Najafian M. A review of α-amylase inhibitors on weight loss and glycemic control in pathological state such as obesity and diabetes[J]. Compar Clin Pathol. 2014;25(2016):1253–64.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
The authors Ai-Qing Yu, Jiong Le, Wen-Tao Huang, Bin Li, Hui-Xin Liang, Qun Wang, Yu-Ting Liu, Charlotte-Aimee Young, Mei-Ying Zhang and Shu-Lan Qin declare that they have no competing interests.
Compliance With Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, AQ., Le, J., Huang, WT. et al. The Effects of Acarbose on Non-Diabetic Overweight and Obese Patients: A Meta-Analysis. Adv Ther 38, 1275–1289 (2021). https://doi.org/10.1007/s12325-020-01602-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01602-9