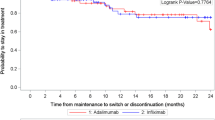
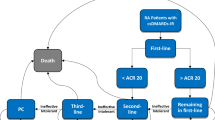
Abstract
Objectives
To assess the cost-effectiveness of reimbursing infliximab for moderate-to-severe Crohn’s disease (MS-CD) in China from the perspective of public insurance payers.
Methods
A decision-analytic model with a lifetime time horizon was constructed to simulate the disease progression and direct medical costs in Chinese MS-CD patients under two scenarios: reimbursing infliximab vs. not reimbursing infliximab. A cross-sectional study and literature review were conducted to estimate model variables. The constructed decision-analytic model ran the base case, one-way sensitivity, and probabilistic sensitivity analyses (PSA) to assess the cost-effectiveness of reimbursing infliximab using reimbursed medical costs.
Results
Base case analysis discounting health benefits and costs estimated that reimbursing infliximab could increase overall survival by 0.604 years, increase total quality-adjusted life years (QALY) by 0.697 QALY, reduce absolute lifetime surgery risk by 13.1%, and increase reimbursed costs by ¥29,409. The incremental cost-effectiveness ratio per gained additional QALY (ICER) based on discounted health benefits and reimbursed medical costs (3% per year) was ¥42,198. The one-way sensitivity analyses identified that the cost-effectiveness of reimbursing infliximab for MS-CD was mainly driven by the treatment efficacies of maintenance therapy, quality of life, and unit price of infliximab. PSA estimated that reimbursing infliximab was associated with a 63.8% chance to be cost-effective under the willingness-to-pay of the 2018 Chinese gross domestic product per capita (GDPPC).
Conclusion
Reimbursing infliximab for MS-CD in Chinese patients was highly attractive, costing Chinese public insurance payers less than the 2018 Chinese GDPPC to gain 1 QALY.


Similar content being viewed by others
References
Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590–605.
Cohen RD. The quality of life in patients with Crohn’s disease. Aliment Pharmacol Ther. 2002;16(9):1603–9.
Feagan BG, Bala M, Yan S, Olson A, Hanauer S. Unemployment and disability in patients with moderately to severely active Crohn’s disease. J Clin Gastroenterol. 2005;39(5):390–5.
Behzadi P, Behzadi E, Ranjbar R. The incidence and prevalence of Crohn’s disease in global scale. SOJ Immunol. 2015;3(2):1–6.
Economou M, Pappas G. New global map of Crohn’s disease: genetic, environmental, and socioeconomic correlations. Inflamm Bowel Dis. 2008;14(5):709–20.
Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Rev Gastroenterol Hepatol. 2006;3(7):390.
Irving PM, Gearry RB, Sparrow MP, Gibson PR. Appropriate use of corticosteroids in Crohn’s disease. Aliment Pharmacol Ther. 2007;26(3):313–29.
Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–9.
Sands BE, Blank MA, Patel K, Van Deventer SJ. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II study. Clin Gastroenterol Hepatol. 2004;2(10):912–20.
Tang DH, Harrington AR, Lee JK, Lin M, Armstrong EP. A systematic review of economic studies on biological agents used to treat Crohn’s disease. Inflamm Bowel Dis. 2013;19(12):2673–94.
Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517.
Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231(1):38.
Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8(4):357–63.
Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–95.
D’Haens G, Baert F, Van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371(9613):660–7.
Suzuki Y, Motoya S, Takazoe M, et al. Efficacy and tolerability of oral budesonide in Japanese patients with active Crohn’s disease: a multicentre, double-blind, randomized, parallel-group Phase II study. J Crohns Colitis. 2013;7(3):239–47.
Tromm A, Bunganič I, Tomsová E, et al. Budesonide 9 mg is at least as effective as mesalamine 4.5 g in patients with mildly to moderately active Crohn’s disease. Gastroenterology. 2011;140(2):425–34.
Yokoyama T, Ohta A, Motoya S, et al. Efficacy and safety of oral budesonide in patients with active Crohn’s disease in Japan: a multicenter, double-blind, randomized, parallel-group phase 3 study. Inflamm Intest Dis. 2017;2(3):154–62.
Singh S, Garg SK, Pardi DS, et al. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn’s disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015;148(1):64–76.
Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145(5):996–1006.
Du XY. Clinical study of surgical treatment for cute abdomen caused by crohn’s disease. China Mod Med. 2011;18(31):36.
Duan M, Li Y, Guo Z, et al. Clinical analysis of acute severe gastrointestinal bleeding in Crohn’s disease. Chin J Gastroenterol. 2018;23(1):38–41.
Feng SC, Cao ZY, Jiang T, et al. Diagnosis and treatment of 29 cases of Crohn’s disease. J Nantong Univ (Medical Sciences). 2010;30(3):187–9.
Lu JY, Ling GL, Qiu HZ, et al. Surgical treatment of the complications of Crohn’s disease. Chin J Mod Oper Surg. 2014;18(01):9–11.
Luan Y, Zong GQ, Chen J, et al. Surgical treatment of Crohn’s disease: an analysis of 19 cases. World Chin J Gastroenterol. 2011;19(17):1851–4.
Niu LY, Tian LJ, Yang JY, et al. Clinical analysis of surgical treatment of Crohn’s disease. Chin Remedies Clin. 2018;18(10):1774–6.
Wang JH, Lin JJ. Clinical effect of surgical treatment of structuring Crohn’s disease. Chin J Dig Surg. 2016;15(12):1160–4.
Xie Y, Dou XT, Yao YL, et al. Experience of urinary tract fistulas complicating Crohn’s disease. Parenter Enter Nutr. 2014;21(3):163–6.
Xue F, Ling F, Feng N, et al. Surgical treatment of Crohn’s disease with free perforation: an analysis of 10 cases. Chin J Inflamm Bowel Dis. 2017;1(3):180–2.
Yang B, Ji JB, Lv CY. Surgical treatment of 10 cases for Crohn’s disease. World Health Dig Med Period. 2011;08(29):10–1.
Zhang GN, Huang SR, Qin QZ, et al. Application of laparoscope in Crohn’s disease diagnosis and treatment. J Minim Invasive Med. 2011;06(4):307–8.
Zhang HB, Han Y, Ling MB, et al. Laparoscopic surgery for the treatment of Crohn’s disease. J Surg Concepts Pract. 2012;17(4):370–3.
Zheng JT, Zhang CY, Li LQ, et al. Risk factors associated with postoperative complications after reoperation for recurrent Crohn disease. Chin J Gastrointestinal Surg. 2011;14(3):181–4.
Zhong MN, Wu B, Niu BZ, et al. Analysis of surgery-related complications and risk factors of ileocolic Crohn’s disease. Chin J Dig Surg. 2016;15(12):1165–9.
Zhong ZQ, Song MM, Bai RX. Analysis of clinical manifestations and emergency operations of Crohn disease: a report for 26 cases. Chin J Postgrad Med. 2009;32(26):20–3.
Zhou W, Xiang JJ, Liu W. Surgical treatment of ileosigmoid fistulas in Crohn’s disease. Chin J Gen Surg. 2016;31(4):322–4.
Zhu XB, Shi S, Ji GH, et al. Clinical analyses for patients of acute abdomen caused by Crohn’s disease. Med J Present Clin. 2015;28(5):1600–1.
Gong J, Wei Y, Gu L, et al. Outcome of surgery for coloduodenal fistula in Crohn’s disease. J Gastrointest Surg. 2016;20(5):976–84.
Huang CQ, Wang DX. Analysis of risk factors associated with poor prognosis and prognosis of colonic and non-colon-type Crohn’s disease. J Gastroenterol Hepatol (Chinese). 2018;27(05):45–9.
Lei XM, Lu L. Clinical characteristics and prognosis analysis of severe Crohn’s disease. J Chengde Med Coll (Chinese). 2017;04:35–7.
Wang M, Ding YB, Xiao WM, et al. Efficacy and safety analysis of long-term application of azathioprine in Crohn’s disease. J Gastroenterol Hepatol (Chinese). 2011;20(7):647–9.
Wang QZ, Wang SJ. Clinical characteristics and survival status of patients with inflammatory bowel disease. China Minkang Medicine (Chinese). 2017;(3):1–3.
Aniwan S, Harmsen WS, Tremaine WJ, et al. Overall and cause-specific mortality of inflammatory bowel disease in olmsted county, minnesota, from 1970 through 2016. Mayo Clin Proc. 2018;93(10):1415–22.
Hovde Ø, Kempski-Monstad I, Småstuen MC, et al. Mortality and causes of death in Crohn’s disease: results from 20 years of follow-up in the IBSEN study. Gut. 2014;63(5):771.
Caini S, Bagnoli S, Palli D, et al. Total and cancer mortality in a cohort of ulcerative colitis and Crohn’s disease patients: the florence inflammatory bowel disease study, 1978–2010. Dig Liver Dis. 2016;48(10):1162–7.
Camus M, Seksik P, Bourrier A, et al. Long-term outcome of patients with Crohn’s disease who respond to azathioprine. Clin Gastroenterol Hepatol. 2013;11(4):389–94.
D’haens G, Reinisch W, Colombel JF, et al. Five-year safety data from ENCORE, a European observational safety registry for adults with Crohn’s disease treated with infliximab [Remicade®] or conventional therapy. J Crohn’s Colitis. 2017;11(6):680–9.
Eshuis EJ, Peters CP, van Bodegraven AA, et al. Ten years of infliximab for Crohn’s disease: outcome in 469 patients from 2 tertiary referral centers. Inflamm Bowel Dis. 2013;19(8):1622–30.
Greener T, Shapiro R, Klang E, et al. Clinical outcomes of surgery versus endoscopic balloon dilation for structuring Crohn’s disease. Dis Colon Rectum. 2015;58(12):1151–7.
Manninen P, Karvonen AL, Huhtala H, et al. Mortality in ulcerative colitis and Crohn’s disease. A population-based study in Finland. J Crohn’s Colitis. 2012;6(5):524–8.
Rönnblom A, Thörn M, Holmström T, Karlbom U, Tanghöj H, Sjöberg D. Clinical course of Crohn’s disease during the first 5 years. Results from a population-based cohort in Sweden (ICURE) diagnosed 2005–2009. Scand J Gastroenterol. 2017;52(1):81–6.
Selinger CP, Andrews J, Dent OF, et al. Cause-specific mortality and 30-year relative survival of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2013;19(9):1880–8.
Wang G, Ren J, Song LIU, et al. Clinical characteristics of non-perianal fistulating Crohn’s disease in China: a single-center experience of 184 cases. Chin Med J. 2012;125(14):2405–10.
Yasukawa S, Matsui T, Yano Y, et al. Crohn’s disease-specific mortality: a 30-year cohort study at a tertiary referral center in Japan. J Gastroenterol. 2019;54(1):42–52.
Kristensen SL, Ahlehoff O, Lindhardsen J, Erichsen R, Jensen GV, Torp-Pedersen C, Nielsen OH, Gislason GH, Hansen PR. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death—a Danish nationwide cohort study. PLoS One. 2013;8(2):e56944.
China Life Insurance Experience Life Table (2010–2013). http://bxjg.circ.gov.cn/web/site0/tab5216/info4054990.htm. Accessed 30 Sept 2019.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–2.
Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess (Winchester, England). 2003;7(27):3–9.
China Statistical Bulletin of National Economic and Social Development. 2018. http://www.stats.gov.cn/tjsj/zxfb/201902/t20190228_1651265.html. Accessed 30 Sept 2019.
Gao X, Yang RP, Chen MH, et al. Risk factors for surgery and postoperative recurrence: analysis of a south China cohort with Crohn’s disease. Scand J Gastroenterol. 2012;47(10):1181–91.
Chow DK, Sung JJ, Tsoi KK, et al. Predictors of corticosteroid-dependent and corticosteroid-refractory inflammatory bowel disease: analysis of a Chinese cohort study. Aliment Pharmacol Ther. 2009;29(8):843–54.
Leong RW, Lee YT, Ching JY, Sung JJ. Quality of life in Chinese patients with inflammatory bowel disease: validation of the Chinese translation of the Inflammatory Bowel Disease Questionnaire. Aliment Pharmacol Ther. 2003;17(5):711–8.
Guo Z, Wu R, Zhu W, et al. Effect of exclusive enteral nutrition on health-related quality of life for adults with active Crohn’s disease. Nutr Clin Pract. 2013;28(4):499–505.
Li J, Liu Q, Chen Y, et al. Treatment patterns, complications, and direct medical costs associated with ankylosing spondylitis in Chinese urban patients: a retrospective claims dataset analysis. J Med Econ. 2017;20(1):91–7.
Lichtenstein GR, Yan S, Bala M, Hanauer S. Remission in patients with Crohn’s disease is associated with improvement in employment and quality of life and a decrease in hospitalizations and surgeries. Am J Gastroenterol. 2004;99(1):91.
Dretzke J, Edlin R, Round J, et al. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-α) inhibitors, adalimumab and infliximab, for Crohn’s disease. Health Technol Assess (Winchester, England). 2011;15(6):1.
Royall RM. The effect of sample size on the meaning of significance tests. Am Statistician. 1986;40(4):313–5.
Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. Criteria for distinguishing effectiveness from efficacy trials in systematic reviews. Rockville: Agency for Healthcare Research and Quality (US); 2006 (Technical Reviews, No. 12.).
Acknowledgements
Funding
This work was supported by Zhejiang Medical and Health Science and Technology project (grant number 2020KY608).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Qian Cao and Wendong Chen formulated the research idea. Haotian Chen, Jihao Shi, Ying Chen, Wendong Chen, and Qian Cao developed the study protocol. Haotian Chen, Jihao Shi, Yipeng Pan, Zhou Zhang, and Hao Fang conducted the patient identification, data extraction, and patient survey of the cross-sectional study in this manuscript. Ying Chen and Wendong Chen conducted the data analysis. Haotian Chen, Jihao Shi, Wendong Chen, and Qian Cao developed the manuscript. All authors have critically reviewed the manuscript and approved this manuscript submission.
Compliance with Ethics Guidelines
Ethics approval for this study was obtained from Sir Run Run Shaw Hospital.
Disclosures
Ying Chen is employed by a consulting firm that receives industry funds to conduct health economics and outcome research. Wendong Chen is employed by a consulting firm that receives industry funds to conduct health economics and outcome research. All other authors (Haotian Chen, Jihao Shi, Yipeng Pan, Zhou Zhang, Hao Fang, Qian Cao) have nothing to disclose.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10050422.
Rights and permissions
About this article
Cite this article
Chen, H., Shi, J., Pan, Y. et al. Cost-Effectiveness of Reimbursing Infliximab for Moderate to Severe Crohn’s Disease in China. Adv Ther 37, 431–449 (2020). https://doi.org/10.1007/s12325-019-01150-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01150-x