Abstract
Ibuprofen first came to market about 50 years ago and rapidly moved to over-the-counter (OTC) sales. In April 2019, the National Agency for the Safety of Medicines and Health Products (ANSM) of France issued a warning for NSAID uses by patients with infectious diseases based on an analysis of 20 years of real-world safety data on ibuprofen and ketoprofen. Nevertheless, ibuprofen remains a mainstay in the analgesic armamentarium and with numerous randomized clinical trials, head-to-head studies, and decades of clinical experience. The authors offer a review of the safety of ibuprofen and how it may differ from other NSAIDs. Ibuprofen is associated with certain well-known gastrointestinal adverse effects that are related to dose and patient population. Among nonsteroidal anti-inflammatory drugs (NSAIDs), ibuprofen has a comparatively low risk of cardiovascular adverse effects. It has been associated with renal and hepatic adverse effects, which appear to depend on dose, concomitant medications, and patient population. The association of ibuprofen with infections is more complex in that it confers risk in some situations but benefits in others, the latter in cystic fibrosis. Emerging interest in the literature is providing evidence of the role of ibuprofen as a possible endocrine disrupter as well as its potential antiproliferative effects for cancer cells. Taken altogether, ibuprofen has a favorable safety profile and is an effective analgesic for many acute and chronic pain conditions, although it—like other NSAIDs—is not without risk. After 50 years, evidence is still emerging about ibuprofen and its unique safety profile among NSAIDs.
Funding
The Rapid Service Fee was funded by Abbott Established Pharmaceuticals Division (EPD).
Similar content being viewed by others
With the passing of Stewart Adams, it is timely for us to review the nearly 50 years of ibuprofen safety. Ibuprofen is one of the world’s most used drugs and remains a mainstay in the analgesia armamentarium. Recent advice as to its adverse impact on infections notwithstanding, ibuprofen remains a “middle of the road” NSAID drug in that it is not strongly selective toward either Cox-1 or Cox-2 |
Ibuprofen is still of great clinical interest; in fact, over 1,200 publications on ibuprofen have appeared since January 2018. Its role in the treatment of many conditions is still being elucidated |
Ibuprofen offers a favorable safety profile compared with other NSAID agents. The most commonly reported adverse events may be described as gastrointestinal and cardiovascular, but their incidence is relatively rare |
The role of ibuprofen in infections is currently being studied. It appears to confer benefits with some infections, such as with cystic fibrosis, but may be detrimental in other cases. However, in nearly 50 years of experience, the role of ibuprofen as a contributory factor in infections has not been demonstrated |
Introduction
In the 1950s, Stewart Adams joined the research department at Boots Pure Drug Co., Ltd., after he had earned degrees in pharmacy at the University of Nottingham and pharmacology from Leeds University [1]. He was tasked with developing a new analgesic for rheumatoid arthritis (RA) with limited side effects and found himself in a modestly equipped postwar British laboratory pitted against much better funded American competitors. At that time, little was understood about the disease processes of RA, and the only drugs used in its treatment were paracetamol (acetaminophen), corticosteroids, and acetylsalicylic acid (ASA)—the mechanism of which was unknown [2]. Adams studied ASA first because it had an anti-inflammatory effect that was not well understood [1], and the anti-inflammatory effect seemed an important advantage over the older drug, paracetamol, first used clinically in 1893 [3]. Adams was initially somewhat reticent about the long-term use of steroids, but he abandoned work on a steroid medication when he found out American drug developers were pursuing this line [2]. Working with organic chemist John Nicholson to elucidate the anti-inflammatory effects of ASA, Adams reviewed small molecules with carboxyl groupings, which led to substituted phenoxypropionic acids and finally propionic acids. By 1958, Adams and Nicholson had already developed over 200 compounds [1] and brought 4 new drugs to clinical trial, none of which offered a clinical benefit over ASA in treating RA. The fifth drug, the first phenylpropionic drug, was finally successful [2]. By 1961, a patent was filed for that related compound 1472, a 2-(-4-isobutylphenyl) propionic acid [4]. Anecdotally, Adams took the very first dose of the new drug himself to help a hangover [1]. Ibuprofen, as it came to be known, was first cleared to market for prescription use in the UK in 1969 (original trade name Brufen, for treating RA) and in the US in 1974. Its safety and tolerability profile allowed the drug to move to over-the-counter (OTC) sales in the UK and US in the 1980s [4]. This versatile new OTC drug was marketed in the UK and was indicated for a variety of pain complaints, including headache.
Despite its established safety record that led to the rapid acceptance of ibuprofen and its relatively rapid migration to OTC sales, it is now being challenged. Recent regulatory advice that there may be serious safety risks associated with ibuprofen challenges its long history of clinical evidence about the relative safety of ibuprofen [5].
The National Agency for the Safety of Medicines and Health Products (ANSM) of France issued a warning in April 2019 about the use of NSAIDs for patients with infectious diseases based on an analysis of 20 years of real-world safety data of ibuprofen and ketoprofen [5]. The warning was based on an analysis of 337 and 49 cases, respectively, over 20 years of infectious complications. Most of the complications were related to Streptococcus and occurred within 2 or 3 days of onset of NSAID therapy. In some of the cases, NSAIDs were administered concomitantly with antibiotics; some were administered by patients themselves without medical advice; other cases involved insect bites, inflammatory lesions, and respiratory conditions. The French regulatory body was concerned that existing infections might be worsened by the use of NSAIDs [6]. It published guidance that NSAIDs were appropriate to use for pain or fever, providing they were used at the minimally effective dose for the shortest possible time. Additionally, NSAID treatment should be discontinued once symptoms have resolved or used no more than 3 days for fever and 5 days for pain. Patients were advised not to take more than one type of NSAID at a time. ANSM also reminded patients that the use of NSAIDs is contraindicated in cases of chickenpox [5]. Our aim is to present a narrative review of the safety history of ibuprofen in light of this recent concern about the drug’s risks.
Methods
In June 2019, keywords in PubMed were searched under guidance of the authors, and the resulting number of articles stated in parentheses were obtained: ibuprofen safety gastrointestinal (223), ibuprofen safety cardiovascular (122), ibuprofen safety renal (111), ibuprofen safety infection (35), and ibuprofen Streptococcus (35). Included were articles about oral ibuprofen involving safety (safety studies and safety and efficacy studies). Systematic reviews and meta-analyses were included as there is a long history of ibuprofen research. The authors were interested in presenting the major safety concerns that have arisen about ibuprofen over the years and present these individually by heading. Excluded were studies on cost effectiveness or non-safety aspects of analgesia; studies exclusively on pediatric, geriatric, or special populations were excluded as were studies not in English or for which we could not obtain a full text. Studies relating to the role of ibuprofen in the treatment of patent ductus arteriosus were excluded. In general, articles published in the past 10 years were given the prime focus. The bibliographies of particularly helpful articles were also searched. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Background information is presented first and then a narrative review of drug safety by condition.
Background of Ibuprofen
The first NSAIDs, like ibuprofen, were nonselective and blocked prostaglandin production synthesized by the cyclooxygenase enzymes COX-1 and COX-2. COX-1 inhibition sometimes led to gastrointestinal (GI) adverse events in some patients. Selective COX-2 inhibitors (coxibs), such as celecoxib, were developed to mitigate these GI adverse events [7], but were later implicated in cardiovascular (CV) side effects [8, 9]. See Fig. 1. While NSAIDs are often described as a drug class, there are important differences among the various NSAIDs in terms of their safety and specific risks for GI, CV, renal, hepatic, and other adverse events [10]. In light of the half-century anniversary of ibuprofen, it is important to emphasize that NSAID safety varies among the many drugs in this class. These selective mechanisms of action are associated with specific risks. Ibuprofen’s balanced selectivity profile between COX-1 and COX-2 helps provide its balanced safety profile.
Ibuprofen is rapidly absorbed by the body but its short half-life necessitates frequent dosing. In healthy subjects, its Tmax is 1.9 ± 1.4 h with a half-life of 2.2 ± 0.4 h [11]. An 800-mg sustained-release (SR) formulation was introduced to allow for more patient-friendly daily dosing (two tablets at once, 1600 mg), which reduces the pill burden. Bioavailability studies with patients dosed with 1600 mg SR ibuprofen once a day showed serum concentrations of the drug equivalent to that achieved with dosing ibuprofen immediate-release (IR) 400 mg formulation four times a day, but with the advantage of avoiding the peaks and troughs associated with divided doses [12, 13]. In a clinical trial comparing both dosing regimens (400 mg IR ibuprofen four times daily versus 1600 mg SR ibuprofen once daily) in patients with RA or osteoarthritis (n = 578), 1600 mg ibuprofen SR once a day provided more effective pain control at 4 weeks than the four-times-daily dose, with 17% of the SR patients reporting an adverse event compared with 20% of the IR patients (p = 0.62) [14].
Overall Studies of Ibuprofen Safety
The paracetamol, aspirin, and ibuprofen new tolerability (PAIN) study evaluated OTC analgesic use by 8677 patients with acute pain and calculated significant adverse events (defined as moderate, serious, or severe, necessitating a second physician consultation or discontinuation of therapy) [15, 16]. The PAIN trial reported that OTC ibuprofen (≤ 1200 mg/day) was similar to paracetamol ≤ 3000 mg/day in the rate of adverse events (13.7% vs. 14.5%) but ibuprofen had significantly fewer events than ASA ≤ 3000 mg/day (13.7% vs. 18.7%, p < 0.001) [15, 16].
The European Medicine Agency (EMA) requested a large head-to-head clinical trial to compare selective and nonselective NSAIDs for better safety data. The PRECISION study enrolled over 24,000 patients in a randomized, multicenter, double-blinded, noninferiority trial, including OA and RA patients. Naproxen was designated as the primary comparator to celecoxib, and ibuprofen was included in the study as well [17]. In broad terms, PRECISION found that celecoxib was associated with a lower risk of GI adverse events than ibuprofen (p = 0.002) and a lower risk of renal adverse events (p = 0.004) [18]. A secondary post hoc analysis of the PRECISION trial examined major NSAID-induced drug toxicity and time to first major adverse event [19]. During the 1–2-year follow-up phase of this large study (n = 24,081), a major NSAID toxicity was reported in 5.3% of all ibuprofen patients (p < 0.001) compared with 4.1% of celecoxib and 4.8% of naproxen patients (p = 0.02). This resulted in a number needed to harm (NNH) of 82 for ibuprofen (95% confidence interval, 53–173) and 135 for naproxen compared with celecoxib [19].
All NSAIDs are associated with some degree of CV risk, but the risk was shown to be greater for coxibs than nonselective NSAIDs such as ibuprofen [9]. The subsequent Adenomatous Polyp Prevention of Vioxx (APPROVE) study found patients with colorectal adenoma treated with rofecoxib had a greater risk of thrombotic CV events [20], and this led to the voluntary withdrawal of rofecoxib from the market in 2004 [21]. The US Food and Drug Administration (FDA) issued a “black-box warning” for all NSAIDs in 2005, which was updated to an enhanced warning in 2015 regarding CV events [22, 23]. Preferred NSAIDs are ibuprofen and naproxen with respect to CV risk [24].
Safety Considerations
All effective drugs have risks as well as benefits, but among OTC NSAIDs ibuprofen has been demonstrated over decades to possess a favorable safety profile [25]. A meta-analysis of ibuprofen safety found that the overall frequency of adverse events reported with ibuprofen patients (n = 1094) was numerically the same or lower than that of adverse events reported by patients who received placebo (n = 1093). Placebo subjects reported significantly more adverse events (31.7%) than ibuprofen subjects (27.4%), p = 0.018, and the frequency of digestive system adverse events was comparable in the placebo and ibuprofen subjects (11.0% and 12.1%, respectively, p = 0.420) [26]. Even compared with paracetamol and ASA, ibuprofen has a favorable safety record; for every 100 patients treated, an additional four will experience adverse events if taking paracetamol instead of ibuprofen, and an additional five suffer adverse events if taking ASA instead of ibuprofen [27]. In an overview of systematic reviews and meta-analyses of various OTC analgesics, ibuprofen’s and other pain relievers’ rates of adverse events were similar to placebo when taken at therapeutic doses for a few days to treat acute pain [28]. The following sections describe specific constellations of adverse events.
Gastrointestinal Safety
The rate of GI adverse events associated with NSAIDs has been the subject of many clinical trials, and rates vary by agents and patient populations. As weak lipid-soluble acids, NSAIDs may interact topically with surface membranes and mucous gel phospholipids [29, 30]. With prolonged use, NSAIDs may become absorbed and accumulate in the mucus membranes to the point that they uncouple mitochondrial oxidative phosphorylation, which, in turn, causes adenosine triphosphate in the cells to decrease, leading to cellular disruption [30, 31]. Repeated ingestion of NSAIDs can compromise mucosal integrity and make mucus membranes more permeable to various potentially noxious agents (e.g., acid), which, in turn, may lead to ulcers [31]. The inhibition of COX-1 is associated with greater stomach acid production, decreased mucus, and depletion of the mucosal tissue of cytoprotective prostaglandins, while the inhibition of COX-2 can inhibit repair and make the mucus membranes more vulnerable to damage [29, 30]. Therefore, all NSAIDs are associated with some degree of risk for upper GI complications [32, 33]. GI complications associated with the use of oral NSAIDs are among the most frequently reported adverse drug events in the US [10]. The relative risk of individual NSAIDs varies with ketorolac and piroxicam associated with the highest risk of GI injury and celecoxib and ibuprofen with the lowest [34] (Fig. 2). Overall, the GI toxicity of ibuprofen is low and similar to placebo at OTC doses [35].
The risk of GI adverse events with NSAIDs may depend on the dose [36] and duration of therapy [34, 37]. In general, short-term use of ibuprofen and other NSAIDs shows GI damage proportional to the acidity of the drug [35] (Fig. 2). With longer-term NSAID therapy (≥ 3 months), endoscopic studies have found ulcer rates ranging from 15 to 35% of patients, although serious outcomes are uncommon [35]. Epidemiologic studies show ibuprofen is consistently ranked lower in toxicity among the NSAIDs while ketorolac ranks consistently high [34]. Specific risk factors for GI symptoms with NSAID use include older age, previous history of bleeding, anticoagulation therapy, and others [35]. Compared with other NSAIDs, the risk of GI adverse events is low with ibuprofen [32, 38,39,40], but the risk of upper GI adverse events associated with ibuprofen increases when taken concomitantly with ASA [41]. In a systematic review of 11 controlled epidemiologic studies comparing ibuprofen with other drugs, ibuprofen ranked lowest or equal to lowest in 10/11 studies for GI risks followed by diclofenac, while azapropazone, tolmetin, ketoprofen, and piroxicam ranked highest. Higher doses of ibuprofen conferred greater relative risks for GI side effects, similar to those associated with naproxen [38]. When that systematic review was subsequently expanded (n = 36 case control studies, 19,648 cases and 105,373 controls, and 8 cohort studies with 400,000 exposed subjects and 1 million non-exposed controls), the unadjusted odds ratio for GI adverse events was 1.81 for ibuprofen (lowest) and 7.46 for piroxicam (highest) [42].
A randomized double-blind trial of 1246 healthy subjects taking 1200 mg/day ibuprofen (maximum OTC dose) or placebo over 10 days reported statistically similar rates of GI adverse events at 19.3% and 16.2% for ibuprofen and placebo, respectively (odds ratio 1.24, 95% confidence interval, 0.90–1.72, p = 0.187) [43]. Overall adverse events (all types) were reported by 44% and 53% in the ibuprofen and placebo groups, respectively [43]. A meta-analysis of eight randomized double-blind placebo-controlled studies of patients administered 800 or 1200 mg/day ibuprofen or placebo reported a similar overall rate of GI adverse events of 12.1% and 11.0% for ibuprofen and placebo, respectively (odds ratio 1.12, 95% confidence interval, 0.85–1.46, p = 0.420) [26]. An analysis of three case-controlled studies of patients with acute upper GI bleeding (n = 2472) versus controls (n = 5877) found the odds ratio of upper GI bleeding with ibuprofen at doses ≤ 1200 mg/day compared with no use of ibuprofen was 1.1. As doses increased from 1200 to 1799 mg/day, the odds ratio increased to 1.8, and the highest doses of ≥ 1800 mg/day had an odds ratio of 4.6 [39]. Thus, at lower doses ibuprofen has a rate of adverse GI events similar to that of placebo, but at higher doses, the rate of adverse GI events increases. This is supported by a study of patients taking prescription ibuprofen, paracetamol, or aspirin for OA or RA that found that serious adverse events among patients who took ibuprofen monotherapy for RA only occurred in patients taking > 1100 mg/day. In the OA group, there were 3.19 GI events per 1000 patient-years for patients who took ibuprofen monotherapy 101–1100 mg/day compared with 9.09 events per 1000 patient-years among those who took > 2200 mg/day [44]. A randomized, blinded, multicenter trial of short-term pain control in patients with painful musculoskeletal conditions (n = 4291) compared ASA, paracetamol, and ibuprofen and found significant adverse events were reported at rates of 15.0% for ibuprofen compared with 20.5% for ASA and 17.0% for paracetamol. Ibuprofen was statistically equivalent to paracetamol and better tolerated than ASA (p < 0.0001). In particular, the rates of GI adverse events were 4.4%, 8.6%, and 6.5% for ibuprofen, ASA, and paracetamol, respectively, with statistically fewer digestive system adverse events for ibuprofen compared with ASA (p < 0.0001) and paracetamol (p < 0.02). All medications were taken at OTC dose ranges for 6 days [45]. In a randomized, double-blind, multiple-dose study of 62 patients with back pain treated with once-daily doses of either ibuprofen SR 1600 mg or diclofenac SR 100 mg over 14 days, ibuprofen SR was more effective, and 16 of the diclofenac patients reported a total of 24 adverse events, of which 8 were deemed definitely related to the study drug compared with 4 ibuprofen patients who reported a total of 9 adverse events of which 3 were deemed definitely related to the study drug (p = 0.002) [13].
The PRECISION clinical trial mentioned earlier was a double-blind controlled study of 24,081 OA or RA patients who required NSAID analgesic therapy [46]. Patients were randomized into one of three groups: celecoxib 100 or 200 mg twice daily, ibuprofen 600–800 mg three times daily, or naproxen 375–500 mg twice daily. Patients were co-prescribed esomeprazole if needed (most patients did) and continued on low-dose aspirin or corticosteroids if already prescribed. Adverse GI events (bleeding, obstruction, perforation, stomach ulcers) were adjudicated blindly. The mean treatment course was 20.3 months with a mean follow-up of 34.1 months. Clinically significant GI events occurred during the treatment course in 0.74% of ibuprofen patients (with a significant difference compared with 0.34% of celecoxib and 0.66% of naproxen patients). The concomitant use of corticosteroids increased total GI events [46]. In the PRECISION study, the NNH for bleeding events from all sites was 417 annually for ibuprofen compared with an NNH of 769 for celecoxib and 625 for naproxen [46]. Chronic iron-deficiency anemia of GI origin was used as an end point for chronic GI injury. Iron-deficiency anemia occurred in 0.41% of celecoxib, 0.80% of ibuprofen, and 0.87% of naproxen patients. The hazard risk for iron-deficiency anemia for celecoxib versus ibuprofen is 0.43 (0.27–0.68, p = 0.0003) [46]. The bleeding risk with OTC ibuprofen is not well studied. A meta-analysis reported the incidence of GI bleeding with OTC ibuprofen is 0–3.19 per 1000 patient-years, and GI-related hospitalizations occurred at a rate of < 0.2% [47]. A large retrospective real-world study included over 3.2 million Americans who used OTC naproxen 220 mg or OTC ibuprofen 200 mg; the index date was set as first mention of the analgesic and data went 365 days prior to index and 90 days post-index. The end point was the occurrence of perforations, ulcers, or bleeds (PUBs). The odds for a PUB event were 1.54 (95% confidence interval, 1.04–2.28, p = 0.03) for naproxen and 1.38 (95% confidence interval, 1.07–1.78, p = 0.01) for ibuprofen. The concomitant use of ASA in either group was associated with a significantly higher risk for a PUB event compared with monotherapy, specifically the odds ratio for ibuprofen plus aspirin was 3.36 (2.36–4.80, p < 00001) and naproxen plus aspirin 2.07 (1.23–3.49, p = 0.005) [48]. The Ibuprofen Paracetamol Study in Osteoarthritis (IPSO) randomized 222 patients to receive ibuprofen 400 mg/three times daily or paracetamol 1000 mg/three times daily over 14 days and found ibuprofen 400 mg at single and multiple doses (1200 mg/day) was a more effective pain reliever than paracetamol 1000 mg at single or multiple doses (3000 mg/day) with a risk for GI adverse events similar to paracetamol, showing a more favorable efficacy/tolerability ratio for ibuprofen over paracetamol over 14 days in knee or hip osteoarthritis [49].
The Italian Pharmacovigilance Network (Rete Nazionale di Farmacovigilanza or RNF) is the database of the Italian Medicine Agency (Agenzia Italiana del Farmaco), which collects adverse drug reaction data. For the period from 2007 to 2011, the RNF collected 2816 reports of adverse drug reactions, of which 13.3% were GI in nature. The combined use of NSAIDs and/or low-dose ASA had the significantly highest association with GI adverse events, and the lowest association with GI events was for their respective monotherapies. NSAIDs associated with GI adverse events were ketorolac (reporting odds ratio 5.6), nimesulide (3.9), diclofenac (3.4), ketoprofen (1.2), and ibuprofen (0.9) [50]. A real-world study was conducted using a case-control model within an historical cohort of patients with first hospitalization for myocardial infarction using the PHARMO drug-dispensing database in The Netherlands. After adjusting for the use of anticoagulants, aspirin, and acetaminophen and adjusting for age, sex, and comorbidities, GI events were almost double among patients currently taking ibuprofen compared with patients who had not had ibuprofen supplied for 60 days or more (odds ratio 1.90, 95% confidence interval, 1.40–2.58) [51]. The European Community addressed the issue of NSAID safety with its Safety of non-Steroidal anti-inflammatory drugs (SOS) collaborative project to develop statistical metrics for NSAID safety [34]. Based on a systematic review of the literature, 28 studies were selected for analysis. The lowest relative risks for GI adverse events occurred in aceclofenac, celecoxib, and ibuprofen, and the highest relative risks were observed for piroxicam, ketorolac, and azapropazone. High daily doses of NSAIDs conferred greater risk (two to three-fold increased RR) of upper GI complications compared with the use of low and medium-range doses (except for celecoxib, which did not exhibit any dose-dependent relationship with GI adverse events). In the SOS analysis, ibuprofen had the lowest range of pooled relative risks for upper GI adverse events [34].
Concomitant use of a proton pump inhibitor (PPI) may help to reduce the risk of GI complications in patients taking nonselective oral NSAIDs, but a retrospective observational study of NSAID-induced gastropathy (n = 62) found that while 66.1% of patients were prescribed PPIs, only 43.9% were taking such medication [52]. Famotidine is a gastroprotective agent that was evaluated in a study of combination ibuprofen 800 mg/famotidine 26.6 mg three times a day to control pain in patients with RA or OA [53]. Pooled results show famotidine significantly reduced the incidence of upper GI adverse events (10.0 vs. 19.5%, p < 0.0001, for younger and 12.9% vs. 26.6%, p = 0.0002, for older patients), gastric events (8.9% vs. 16.8%, p = 0.0004, for younger and 11.9% vs. 23.4%, p = 0.0011, for older patients), and duodenal ulcers (1.1% vs. 5.4%, p < 0.0001, for younger and 1.0% vs. 4.5%, p = 0.0096, for older patients), where younger patients were < 60 years and older patients ≥ 60 years. Therefore, the combination therapy of ibuprofen plus famotidine reduced GI ulcers by 51% in younger and 59% in older patients [53]. The risk of upper GI ulcers was reduced by 44% with the combination therapy compared with ibuprofen alone [54]. One-year safety results confirmed a favorable tolerability profile with respect to GI events [55]. The Registration Endoscopic Studies to Determine Ulcer Formation of HZT-501 Compared with Ibuprofen: Efficacy and Safety Studies (REDUCE-1 and REDUCE-2 trials) found in a pooled analysis of the two studies that there were significantly fewer gastric ulcers (12.5%) and duodenal ulcers (1.1%) with the famotidine-ibuprofen combination compared with ibuprofen alone (20.7% and 5.1%, respectively) [56].
There are known risk factors for GI adverse events with NSAIDs, including but not limited to ibuprofen. Older age confers risk; in the PRECISION study, more patients ≥ 63 years had a clinically significant GI event than younger patients. Clinically significant GI events occurred in 0.33% of patients aged < 63 years compared with 0.79% for patients aged > 63 years (p < 0.0001) [46]. Other risk factors are a history of upper GI bleeding and perforation [57], non-Caucasian origin, male sex [58], and the concomitant use of corticosteroids [46].
Cardiovascular Safety
The CV risk of NSAIDs is thought to be inhibition of prostaglandin production in the renal system, increasing blood pressure, due to fluid overload placing the patient at elevated risk for a CV adverse event [59]. The risk is greater to patients with cardiac conditions, such as chronic heart failure [60]. In 2015, the FDA stated that the evidence was insufficient to support differentiating claims among NSAIDs with respect to their CV risk [23]. Most of the evidence of the CV risk of NSAID therapy comes from controlled trials of prescription NSAIDs, and there is a paucity of evidence about OTC ibuprofen and even ibuprofen in general, such that the CV risk conferred by ibuprofen is somewhat disputed [61, 62]. Among the nonselective NSAIDs, ibuprofen is associated with less CV risk than diclofenac [10].
In a retrospective study of OA patients from a Danish database (n = 533,502), 64.3% of all patients had received a prescription NSAID, and 7.2% had experienced a CV event during follow-up. The hazard ratios for the composite end point of CV death, nonfatal MI (myocardial infarction), or nonfatal ischemic stroke or transient ischemic attack for the various NSAIDs compared with non-use of an NSAID were: 1.90 rofecoxib (95% confidence interval, 1.74–2.08), 1.47 celecoxib (95% confidence interval, 1.34–1.62), 1.44 diclofenac (95% confidence interval, 1.36–1.54), 1.20 ibuprofen (95% confidence interval, 1.15–1.25), and 1.20 naproxen (95% confidence interval, 1.04–1.39). With celecoxib as the reference, the hazard ratio for the composite end point for ibuprofen was 0.81 (95% confidence interval, 0.74–0.90), the same as for naproxen (0.81, 95% confidence interval, 0.68–0.97) [63].
In the PRECISION study, 24,081 OA or RA patients were randomized and assigned to one of three groups: celecoxib 100–200 mg/day, ibuprofen 600–800 mg three times a day, or naproxen 375–500 mg twice a day. Celecoxib was found to be noninferior to ibuprofen or naproxen with respect to CV safety [18]. The primary composite end point was CV death (including hemorrhagic death), nonfatal MI, or nonfatal stroke. In the intention-to-treat analyses, this primary end point was achieved by 2.3%, 2.5%, and 2.7% of the celecoxib, naproxen, and ibuprofen patients, respectively. In on-treatment analysis, the primary end point was met by 1.7%, 1.8%, and 1.9% of the celecoxib, naproxen, and ibuprofen groups, respectively (p < 0.001 for non-inferiority comparisons for celecoxib vs. naproxen and for celecoxib vs. ibuprofen) [18]. It has been recommended based on this trial that patients with CV risk factors avoid NSAIDs, if possible, or take the lowest effective dose for the shortest period of time if NSAID therapy must be used [64]. As 68.8% of PRECISION patients discontinued the study drug, nonadherence may have affected results and must be viewed as a study limitation [18].
The Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET) compared lumiracoxib 400 mg/day with ibuprofen 800 mg three times a day and naproxen 500 mg twice daily. Least-squares mean change from baseline to week 4 for systolic blood pressure was + 0.57 mmHg for lumiracoxib versus + 3.14 mmHg for ibuprofen (p < 0.0001) [65]. Ibuprofen was also associated with a significant increase in systolic blood pressure in the PRECISION Ambulatory Blood Pressure Measurement (ABPM) sub-study compared with celecoxib for a − 3.9 mmHg differential between celecoxib and ibuprofen at 4 months (n = 444, p = 0.0009). The patient population with normal blood pressure at baseline who developed hypertension (defined as systolic ≥ 130 and/or diastolic ≥ 80 mmHg) was largest in the ibuprofen group (23.2%) followed by 19.0% naproxen and 10.3% celecoxib (odds ratio 0.39, p = 0.004, and odds ratio 0.49, p = 0.03, for ibuprofen and naproxen, respectively) [66]. The PERFORM study was two nested case-control analyses (2153 cases with a major CV event during the follow-up and 4306 matched controls plus 809 major bleeding cases matched to 1616 controls for separate analyses). Overall, 2.5% of patients in this study were prescribed ibuprofen versus 12.3% prescribed paracetamol. Paracetamol but not ibuprofen was associated with the risk of a major adverse cardiac event (MACE), odds ratio 1.21 (95% confidence interval, 1.04–1.42), or major bleeding, odds ratio 1.60 (95% confidence interval, 1.26–2.03). Time-varying analysis found the risk for MACE increased for both drugs with duration of therapy; the risk of major bleeding increased only with paracetamol [67].
In a large meta-analysis of 280 placebo-controlled clinical trials plus 474 of head-to-head NSAID trials (68,342 and 165,456 person-years, respectively), it was found that, compared with placebo, major vascular events occurred significantly more often with a coxib (rate ratio 1.37, 95% confidence interval, 1.14–1.66, p = 0.0009) and diclofenac 150 mg/day (rate ratio 1.41, 95% confidence interval, 1.12–1.78, p = 0.0036). Ibuprofen 2400 mg/day versus placebo increased major coronary events (rate ratio 2.22, 95% confidence interval, 1.10–4.48, p = 0.0253) but not major vascular events (rate ratio 1.44, 95% confidence interval, 0.89–2.33, p = 0.14) [7]. Stroke risk has been evaluated with ibuprofen with equivocal results. The aforementioned meta-analysis did not find that ibuprofen or any NSAID significantly increased the risk of stroke, but a meta-analysis by Trelle and colleagues of 31 studies (115,000 patient-years) did [68]. In a longitudinal cohort study, stroke risk was found somewhat elevated for patients taking prescription doses of ibuprofen (standardized mortality ratio of 1.10, 95% confidence interval, 1.0–1.3) for hemorrhagic stroke and 1.18 (95% confidence interval, 1.1–1.3) for other stroke, but this study did not examine OTC ibuprofen use [69]. A longitudinal cohort study from the Pennsylvania Medicare database could not find an association between ibuprofen and stroke (rate ratio 0.95, 95% confidence interval, 0.78–1.16) [61]. A network meta-analysis of 31 trials (n = 116,429) found ibuprofen was associated with the highest risk of stroke (3.36, 1.00–11.6) while rofecoxib was associated with the greatest risk of MI (2.12, 1.26–3.56), and etoricoxib and diclofenac were associated with the highest risk for CV death [68]. In a propensity-matched study exploring the risk of acute coronary syndrome from a French nationwide database that matched 315,269 treatment episodes of ibuprofen (n = 168,400 patients) to 630,457 paracetamol episodes (n = 395,952 patients), no evidence of increased risk of acute coronary syndrome was found in patients treated with ibuprofen compared with paracetamol, despite a transient increase in coronary events in the first 2 weeks for ibuprofen users (hazard ratio 1.70, 95% confidence interval, 1.11–2.59). Similar results were observed for paracetamol and ibuprofen at 3 months [70].
In a large population-based cohort study from Taiwan, 55,629 hypertensive patients who took any of several NSAIDs were evaluated in terms of major CV events, defined as first hospitalization for ischemic stroke, acute MI, congestive heart failure, transient ischemic attack, unstable angina, and coronary revascularization. Patients were followed on an as-treated basis for up to 28 days after index date to the following event: outcome occurred, index NSAID discontinued, change in NSAID therapy, date of hospital discharge, outpatient visit, or visit to a community pharmacy [71]. In this patient population, 65% were taking celecoxib, 15% ibuprofen, 35% etoricoxib, and 34% diclofenac. The incidence rate was 122 per 1000 person-years for selective NSAIDs compared with 76 per 1000 person-years for nonselective NSAIDs. In this study, the mean daily dose of ibuprofen was 1084 mg compared with 210 mg/day for celecoxib and 107 mg/day diclofenac. It should also be noted that unlike many other studies of NSAIDs, doses were relatively low and duration of therapy short (28 days) [71]. The Celecoxib Long-Term Arthritis Safety Study (CLASS) database evaluated higher doses of celecoxib and therapeutic-range doses of ibuprofen and diclofenac in a population of 8059 OA or RA patients. Patients received celecoxib 400 mg twice a day, ibuprofen 800 mg three times a day, or diclofenac 75 mg twice a day. Celecoxib had a similar rate of hypertension or edema compared with diclofenac but a significantly lower one than ibuprofen. More ibuprofen than celecoxib patients initiated antihypertensive therapy [72].
While there is a clear association of acute myocardial infarction (AMI) with coxibs, the association of nonselective NSAIDs, such as ibuprofen, with AMI is less apparent. A meta-analysis confirmed that as a class, nonselective NSAIDs were associated with a relative AMI risk of 1.19 (95% confidence interval, 1.08–1.31), and the risks specifically for ibuprofen and diclofenac were 1.11 and 1.38, respectively (95% confidence interval for both, ranges 1.06–1.17 and 1.22–1.57, respectively) [73]. In general, NSAIDs, even traditional nonselective NSAIDs such as ibuprofen, pose a risk for patients with a history of MI even with short-term use, but this risk was lower for ibuprofen than for diclofenac and the COX-2 selective inhibitors [74]. High-dose nonselective NSAIDs have been shown to be associated with increased mortality rates among patients with a prior MI [hazard ratio for ibuprofen 1.50, 1.36–1.67 compared with 2.80 (2.41–3.25) for rofecoxib and 2.40 (2.09–2.80) for diclofenac] [75]. In a retrospective study of 3859 patients who received both ASA and ibuprofen (52,139 patient-months of use) compared with 10,239 patients who took ASA monotherapy (156,419 patient-months), there were 138 (ASA and ibuprofen) and 684 instances (ASA only) of MI, respectively, showing that adding ibuprofen to ASA therapy did not increase the risk for MI compared with ASA alone [76].
Patients with known coronary disease may be at elevated risk for CV adverse events during NSAID therapy, with moderate risk described for ibuprofen compared with diclofenac (higher risk) and naproxen (lower risk with significant results only for treatment > 90 days) [77]. The degree to which individual risk factors play a role in CV risk emerged in a study of various NSAIDs in 16,326 Taiwanese patients treated > 180 days with ibuprofen, etodolac, nabumetone, or naproxen [78]. In this study, the overall prevalences of AMI, angina, cerebrovascular attack, and transient ischemic attack were significantly higher in those with a history of CV disease than in those without such a history and without pre-existing conditions such as hypertension, dyslipidemia, diabetes, congestive heart failure, and chronic renal disease. In fact, a history of CV disease was the single most significant determinant of CV events in these patients. The four NSAID agents studied all had similar CV risks [78]. In many cases, individual risk factors for CV disease and the patient’s overall health status may determine CV risk to a greater extent than the drug itself [79].
In patients without specific CV risks, ibuprofen at 2400 mg/day could slightly increase the risk for coronary events. It should be noted that ibuprofen at doses of 1200 mg/day may decrease the cardioprotective benefits of ASA [80]. Overall, low-dose ibuprofen (1200 mg/day) and low-dose naproxen (1000 mg/day) appear to have the most favorable thrombotic CV profile among the NSAIDs [80].
Renal Safety
The kidneys produce prostacyclin and prostaglandin E2 (PGE2), and it is thought that many NSAIDs affect the homeostasis of these renal prostaglandins by inhibiting COX-1 and/or COX-2 [15]. The renal prostaglandins promote vasodilatation which, in turn, promotes renal blood flow [80]. In euvolemic patients, NSAIDs do not cause significant renal effects, but as patients age and kidney function declines, higher-than-anticipated free levels of the NSAID and a prolonged half-life and thus a more marked inhibition of prostaglandin synthesis could be observed than would be expected from a similar dosage in a healthy person. Thus, the dose of the NSAID should be adjusted for this patient population [80].
Renal prostaglandins (PGI2 and PGE2) modulate the secretion of renin, sodium, potassium, and water reabsorption [81]. COX-1 regulates the hemodynamics of the kidney system and controls glomerular filtration, while COX-2 helps to control excretion of salt and water [82]. Thus, prostaglandin synthesis inhibition may result in acute kidney injury, hyperkalemia, peripheral edema, hypertension, weight gain, and other symptoms [15]. NSAIDs may also interfere with antihypertensive therapy, and caution should be exercised in patients taking an NSAID plus medication for high blood pressure [83]. NSAIDs, including but not limited to ibuprofen, may not be appropriate to use in geriatric patients with chronic kidney disease or heart failure [84]. The renal risks associated with NSAIDs are rare but several: retaining sodium, peripheral edema, increased blood pressure, weight gain, congestive heart failure, hyperkalemia, and acute renal failure [82]. Patients suffering dehydration are at elevated risk for a drug-associated renal adverse event from any NSAID [85]. In a systematic review of NSAID safety, ibuprofen had the highest rate of renal complications for treating hip and knee arthritis (compared with naproxen, diclofenac, and celecoxib) with an odds ratio of 2.32 (range 1.45–3.71) [86]. A cross-sectional study of 802 hip arthroscopy patients taking NSAIDs either alone or concomitantly with diuretics and/or an angiotensin-converting enzyme (ACE) inhibitor found NSAID use (any NSAID) had only a slight association with renal dysfunction (odds ratio 1.4, 95% confidence interval, 0.9–2.2) but was more likely to occur with NSAIDs having a half-life ≥ 4 h (odds ratio 2.6, 95% confidence interval, 1.2–5.7). A higher risk of renal impairment was observed in patients who took a diuretic concomitantly with an NSAID (odds ratio 3.7, 95% confidence interval, 1.7–8.3) and indeed in those who took diuretics even without an NSAID (odds ratio 3.5, 95% confidence interval, 1.6–7.6).
The Celecoxib Long-Term Arthritis Safety Study (CLASS) mentioned earlier (n = 8059 study of celecoxib compared with ibuprofen and diclofenac) reported that changes in serum creatinine clearance occurred in similar numbers of celecoxib and ibuprofen patients. In the subpopulation of patients with mild pre-renal azotemia, fewer celecoxib patients had reduced renal function (3.7%) compared with diclofenac patients or ibuprofen patients (7.3%, p < 0.05 for both) [72]. In a case-control study based on Tennessee Medicaid patients (n = 11,698), ibuprofen had no association with increased risk of acute renal failure at lower OTC doses but did confer a risk at higher doses (adjusted odds ratios were 0.94, 1.89, and 2.32 at ≤ 1200 mg/day, between 1200 and 2400 mg/day, and ≥ 2400 mg/day, respectively) [87]. In the PRECISION study described earlier, the risk of renal adverse events was significantly lower in celecoxib than ibuprofen patients (p = 0.004) but the risk was similar between celecoxib and naproxen (p = 0.19) [18].
Hepatic Safety
While drug-related liver damage is one of the most serious and concerning of all drug reactions, the incidence of liver toxicity is quite low with ibuprofen [88, 89]. As ibuprofen has a long history of widespread use for a variety of conditions, the low reported rate of liver toxicity suggests that it is rare with ibuprofen use, likely because of its short plasma half-life of 1.8–2.0 h and its lack of a pathologic metabolite [89]. In a systematic review of randomized clinical trials of NSAID use, none of the NSAIDs studied (including ibuprofen) exhibited an increase in the rate of liver-related serious adverse events, hospitalizations, or deaths [90].
In a case-control study at several centers in Italy conducted from October 2010 to January 2014, 179 cases of acute liver injury were matched to 1770 controls who had acute complaints that did not involve the liver. Overall, the adjusted odds ratio for an acute serious liver injury to have an association to an NSAID was 1.69 (95% confidence interval, 1.21–2.37) with risk heightened by prolonged length of exposure and higher doses. The risk for hepatotoxicity was 1.92 for ibuprofen (95% confidence interval, 1.13–3.26) at the recommended dosage and 3.73 at higher doses (95% confidence interval, 1.11–12.46). By comparison, the risk for ketoprofen at doses ≥ 150 mg was 4.65 (95% confidence interval, 1.33–10.00) [91]. In this study, nimesulide and ibuprofen were associated with a significantly increased risk of liver damage (adjusted risk of 2.10, 95% confidence interval, 1.28–3.47 and 1.92, 95% confidence interval, 1.13–3.26, respectively), while paracetamol increased the risk of hepatotoxicity three-fold (adjusted odds ratio of 2.97, 95% confidence interval, 2.09–4.21) [91]. Preliminary results from a clinical trial of patients admitted to hospital for acute liver injury (n = 63) found that 13 had prior exposure to NSAIDs and 24 to paracetamol (non-overdose). The per-patient risk for liver injury for ibuprofen was 19.5 (range 5.31–49.9) per million users compared with 58.0 per million for paracetamol (37.2–86.3) [92].
Acute liver failure leading to transplant (ALFT) was evaluated in a multicenter, multinational study of 9479 patients registered for transplant, of whom 600 were scheduled for an ALFT. Of the ALFT patients, 301 had drug exposure in the past 30 days, of which 40 had taken an NSAID. The event rate per million-treatment-years was 1.59 for all NSAIDs pooled together (95% confidence interval, 1.1–2.2) and 2.3 (95% confidence interval, 1.2–3.9) for ibuprofen versus 3.3 for paracetamol (95% confidence interval, 2.6–4.1) without overdose and 7.8 (95% confidence interval, 6.8–9.0) with overdose. The NSAIDs used in this study (90 days before first symptoms) were celecoxib (n = 2), diclofenac (n = 7), etodolac (n = 2), ibuprofen (n = 14), indomethacin (n = 1), ketoprofen (n = 3), ketorolac (n = 2), meloxicam (n = 1), naproxen (n = 2), niflumic acid (n = 1), nimesulide (n = 9), and “unspecified NSAID” (n = 3). Of the seven cases reporting the use of diclofenac, one was for a topical product. Thus, ALFT following NSAID use was rare, and the rate of non-overdose paracetamol liver failure was more than twice that of NSAID-related liver failure. Event rates for NSAIDs per million-treatment-years (95% confidence interval for all) were 2.28 for ibuprofen (1.21–3.90), 2.16 for celecoxib (0.26–7.79), 1.55 for diclofenac (0.57–3.38), 1.63 for naproxen (0.20–5.89), and 19.44 for ketorolac (2.33–70.26), which was the highest event rate observed for an NSAID [93].
Infections
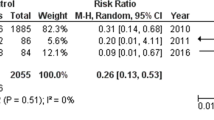
The European Society for Clinical Microbiology and Infectious Diseases has recommended either ibuprofen or acetaminophen for the relief of sore throat symptoms in its Sore Throat Guidelines [94]. Group A Streptococcal (GAS) infections may sometimes lack an apparent portal for bacterial entry. A study of varicella compared 52 pediatric cases of invasive GAS infections with 172 controls and reported that nonselective NSAIDs, in particular ibuprofen, did not significantly increase the risk of necrotizing GAS infections but observed a significant association between non-necrotizing invasive GAS infections and ibuprofen use [95]. The use of nonselective NSAIDs in an animal study showed the agents diminished the effectiveness of antibiotic therapy in mice given a sublethal intramuscular dose of GAS, while COX-selective NSAIDS had no significant effects [96]. NSAIDs inhibit leukocyte-mediated host defense mechanisms, suppress fever, and increase cytokine production (TNF-α, specifically) involved in septic shock, and they mask the clinical signs of infection and promote an overproduction of cytokine. NSAIDs may therefore delay treatment, facilitate local spread of infection, and predispose patients to shock or organ failure [97]. Study results to date have been equivocal with reports of a high incidence of NSAID use in streptococcal toxic shock syndrome (STSS) patients but not based on controlled data assessing any cause and effect on this matter [98,99,100]. An epidemiologic study from the UK found STSS was independently associated with NSAID use with a three-fold increase of STSS in patients who used NSAIDs (odds ratio 3.00, 95% confidence interval, 1.30–6.93, p = 0.01), but as no data were collected about time, dose, indications for use, or which agent was taken, a causal link between the use of NSAIDs and STSS cannot be inferred from this study [101].
Ibuprofen and other NSAIDs are sometimes used to treat symptoms of colds and flu (sore throat, fever, myalgia, headache, sinus pain, and so on). Ibuprofen may be administered to children in cough syrup or cold medicines. In a double-blind randomized study comparing ibuprofen (doses ≤ 1200 mg/days) with ASA and paracetamol (≤ 3000 mg/days for each) in 2815 patients with symptoms of a cold, flu, or sore throat (CF/ST), significant adverse events were reported in 12.0%, 15.7%, and 12.3% of ibuprofen, ASA, and paracetamol patients, respectively, and ibuprofen was significantly better tolerated than ASA (p = 0.02) with a tolerability similar to that observed with paracetamol [102]. A retrospective review found short courses of ibuprofen (as well as paracetamol and other NSAIDs) were often used to treat upper respiratory tract infections although there are few randomized clinical trial data on the safety and effectiveness of ibuprofen in that setting. Despite limited data, it appears from real-world experience that ibuprofen at OTC doses is safe for the treatment of symptoms of cold and flu, and there is no evidence that ibuprofen or analgesics prolong the course of colds and flu by an effect on the immune system or by reducing fever [103]. Murine studies found that nonselective NSAIDs can increase GAS infections of injured muscles and exacerbate established infections [104, 105]. On the other hand, the use of ibuprofen in a gerbil study of penicillin-resistant pneumococcal acute otitis media found ibuprofen combined with antibiotic therapy improved outcomes [106].
Ibuprofen has been used in the treatment of cystic fibrosis, a condition characterized by chronic inflammation and infection. Infections associated with cystic fibrosis tend to be polymicrobial and provoke acute inflammatory response with an abundance of neutrophils, challenging the ability of the pulmonary system to clear them [107]. Thus, cystic fibrosis sets up a vicious cycle of infection, airway inflammation, and airway obstruction. Ibuprofen along with other NSAIDs and inhaled corticosteroids is sometimes used to help address the inflammation [107, 108]. A recent study proposed that part of ibuprofen’s effectiveness in this setting occurs because ibuprofen has an antimicrobial effect against Pseudomonas aeruginosa and Burkholderia bacteria associated with cystic fibrosis [108]. Ibuprofen reduced the growth rate and bacterial burden of these bacteria in a dose-dependent fashion in an acute pseudomonas pneumonia mouse model [108]. A long-term clinical trial has found that ibuprofen may slow the progression of cystic fibrosis lung disease in children with ibuprofen-treated patients experiencing a 40% slower rate of decline compared with placebo (p = 0.02) [109]. Other studies have suggested the antimicrobial effects of ibuprofen in cystic fibrosis [109,110,111].
Bleeding Risk
Bleeding during plastic surgery often causes plastic surgeons to withhold NSAIDs in favor of other analgesic agents, such as tramadol. In a systematic review and meta-analysis (four high-quality randomized clinical trials of procedures involving face, breast, hernia repair, and Mohs surgery, n = 443), ibuprofen was not associated with an increased risk of bleeding and was found to provide effective pain relief as well [112]. In this study, ibuprofen was started either immediately preceding the surgery or in the post-anesthesia care unit and continued up to a week after surgery.
Hypersensitivity
Hypersensitivity is associated with an idiosyncratic type B drug reaction that can occur in susceptible patients and may be described as a reaction that includes fever and rash and involves internal organs. Hypersensitivity affects numerous drugs and can be treatment limiting. Although hypersensitivity reactions are rare, NSAIDs have been implicated in such cases, with the most frequent diagnosis being urticaria/angioedema with cross tolerance [113,114,115]. Since these reactions are so rare, there are few studies to quantify their incidence or incidence by specific NSAID type. In a retrospective database study, it was reported that there were no cases of NSAID hypersensitivity among 24,500 patient-years of experience with ibuprofen compared with none in 14,000 patients-years for naproxen [116]. It typically commences within the first 12 days of treatment but may begin as early as the first dose. Ibuprofen hypersensitivity has been described in the literature and is a host-dependent drug reaction that likely involves an interplay of metabolic and immunologic factors [117].
Discussion
Ibuprofen is a well-established medication with 5 decades of real-world clinical experience and robust scientific data, which—taken together—have shown it to be a versatile and effective analgesic with a long-established and strongly supported safety profile. Taken as directed in the therapeutic dose range, ibuprofen is associated with significant anti-inflammatory action, effective analgesia, and a comparatively low risk of GI, CV, renal, hepatic, or infectious side effects. In fact, ibuprofen has a favorable profile in terms of safety and effectiveness compared with other similar agents. While NSAIDs are often described or treated as a broad class of drugs, the safety profiles of these analgesics differ, and ibuprofen emerges as a drug with favorable safety attributes.
This wealth of clinical experience has also suggested that ibuprofen may have other effects. There is emerging evidence that ibuprofen may in certain specific situations act as an endocrine disrupter [118, 119]. The role of ibuprofen in cancer is currently being discussed in the literature, because ibuprofen offers antiproliferative benefits in some situations [120,121,122]. Thus, the discussion about infection and ibuprofen is not surprising as we continue to learn more about this molecule in specific settings with specific patient populations. In this connection, it must be pointed out that ibuprofen seems to be beneficial for pediatric cystic fibrosis patients [108]. Therefore, further study is warranted as are more in-depth discussions and greater gathering of evidence.
Ibuprofen has been a mainstay of our analgesic armamentarium. It goes without saying that the safety and safe use of analgesics is of utmost concern to prescribers, but clinicians must take a balanced view by evaluating the evidence and weighing risks and benefits for each individual patient in each unique case, and even consider combination drug therapy for the appropriate treatment of at-risk patients where a tailored therapy is absolutely necessary [123, 124]. The importance of ibuprofen to clinical practice can be seen in the volume of research interest in this product: the PubMed database shows that over 1200 articles have been published on this “old drug” in the year 2018 to date. As we learn more, new risks but also new benefits come to light. For most clinicians on the frontlines of the healthcare system—the men and women who regularly treat patients with various acute and chronic pain syndromes—ibuprofen must be considered one of the comparatively safer effective analgesics.
Conclusions
In the last half-century, ibuprofen has earned a place in the analgesic armamentarium as a versatile analgesic product with a favorable safety profile. Its pharmacologic properties and COX-selectivity (neither strongly COX-1 nor COX-2) have caused it to rank among the safest of the NSAID pain relievers. Risks for GI adverse events, CV side effects, renal, and hepatotoxic effects are very low with ibuprofen compared with other NSAIDs. While NSAIDs all provide effective pain control for many types of painful conditions, such as RA, osteoarthritis, back pain, headache, and others, safety aspects of NSAIDs must be considered. NSAIDs are not all the same when it comes to safety profiles. Clinicians must always try to balance benefit against risk with NSAIDs and, indeed, all medications. While ibuprofen may not be appropriate for all patients, clinicians should evaluate the evidence and safety when making prescribing choices or recommending OTC products to their patients.
References
Ferry G. Stewart Adams obituary. The Guardian. 2019. https://www.theguardian.com/science/2019/feb/13/stewart-adams-obituary. Accessed 4 Jun 2019.
An interview with Stewart Adams. Cell Press. Trends in Pharmacological Sciences Web site. 2012. https://www.cell.com/trends/pharmacological-sciences/pdf/S0165-6147(11)00194-5.pdf. Accessed 4 Jun 2019.
Prescott LF. Paracetamol: past, present, and future. Am J Ther. 2000;7(2):143–7.
BBC News. Ibupforen: Dr Stewart Adams who helped discover drug dies at 95. BBC. England Web site. 2019. https://www.bbc.com/news/uk-england-nottinghamshire-47073913. Accessed 4 Jun 2019.
ANSM. [Anti-inflammatoires non stéroïdiens (AINS) et complications infectieuses graves—Point d’Information]. Agence nationale de securite du medicament et des produits de sante. 2019. https://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Anti-inflammatoires-non-steroidiens-AINS-et-complications-infectieuses-graves-Point-d-Information. Accessed 4 Jun 2019.
Taylor N. France’s ANSM warns about NSAIDs following safety review. Regulatory Affairs Professionals. EU Regulatory Roundup Web site. 2019. https://www.raps.org/news-and-articles/news-articles/2019/4/eu-regulatory-roundup-frances-ansm-warns-about-n. Accessed 4 Jun 2019.
Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–79.
Patrono C. Cardiovascular effects of cyclooxygenase-2 inhibitors: a mechanistic and clinical perspective. Br J Clin Pharmacol. 2016;82(4):957–64.
Varrassi G, Pergolizzi J, Peppin J, Paladini A. Analgesic drugs and cardiac safety. In: S. Govoni et al, editors. Brain and Heart Dynamics. Switzerland. Springer; 2019. https://doi.org/10.1007/978-3-319-90305-7_43-1.
Pelletier JP, Martel-Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S22–7.
Albert KS, Gernaat CM. Pharmacokinetics of ibuprofen. Am J Med. 1984;77(1):40–6.
Bratty J, Rotherham N, Underwood L. Comparative bioavailability of ibuprofen from two Brufen Retard tablets 80 mg as a single dose and from Brufen immediate release tablets 400 mg taken four times a day. In: Maddison P, ed. New developments in the management of chronic arthritis. Highlights of an international symposium Held in Rome, Italy. vol 41. Theale, Berkshire, United Kingdom: Colwood House Medical Publications (UK) Ltd.; 1991.
Driessens M, Famaey J-P, Orloff S, et al. Efficacy and tolerability of sustained-release ibuprofen in the treatment of patients with chronic back pain. Curr Ther Research. 1994;55(11):1283–92.
O’Connor T, Anderson A, Lennox B, Muldoon C. A novel sustained-release formulation of ibuprofen provides effective once-daily therapy in the treatment of rheumatoid arthritis and osteoarthritis. Brit J Clin Pharmacol. 1993;47(1):10–3.
Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug–drug interactions with over-the-counter NSAIDs. Ther Clin Risk Managem. 2015;15(11):1061–75.
Moore N, Van Ganse E, Le Parc JM, et al. The PAIN study: paracetamol, aspirin and ibuprofen new tolerabiity study. Clin Drug Investig. 1999;18(2):89–98.
Patrono C, Baigent C. Coxibs, traditional NSAIDs, and cardiovascular safety post-precision: what we thought we knew then and what we think we know now. Clin Pharmacol Ther. 2017;102(2):238–45.
Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375(26):2519–29.
Solomon DH, Husni ME, Libby PA, et al. The risk of major nsaid toxicity with celecoxib, ibuprofen, or naproxen: a secondary analysis of the precision trial. Am J Med. 2017;130(12):1415–1422.e1414.
Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–102.
Sibbald B. Rofecoxib (Vioxx) voluntriy withdrawn from market. Can Med Assoc J. 2004;171(8):1027–8.
FDA. CΟΧ-2 selective (includes Bextra, Celebrex, and Vioxx) and Non-Selective Non-Steroidal Anti-Inflammatory Drugs. Food and Drug Administration. Drug Safety and Availability Web site. 2005. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/cox-2-selective-includes-bextra-celebrex-and-vioxx-and-non-selective-non-steroidal-anti-inflammatory. Accessed 11 Jun 2019.
FDA. FDA Drug Safety Communication: FDA strenghtens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. Food and Drug Administration. Drug Safety and Availability Web site. 2015. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-warning-non-aspirin-nonsteroidal-anti-inflammatory. Accessed 11 Jun 2019.
Thomas D, Ali Z, Zachariah S, Sundararaj KGS, Van Cuyk M, Cooper JC. Coxibs refocus attention on the cardiovascular risks of non-aspirin NSAIDs. Am J Cardiovasc Drugs. 2017;17(5):343–6.
Moore A, Crossley A, Ng B, Phillips L, Sancak O, Rainsford KD. Use of multicriteria decision analysis for assessing the benefit and risk of over-the-counter analgesics. J Pharm Pharmacol. 2017;69(10):1364–73.
Kellstein DE, Waksman JA, Furey SA, Binstok G, Cooper SA. The safety profile of nonprescription ibuprofen in multiple-dose use: a meta-analysis. J Clin Pharmacol. 1999;39(5):520–32.
Moore N. Forty years of ibuprofen use. Int J Clin Pract Suppl. 2003;135:28–31.
Moore RA, Wiffen PJ, Derry S, Maguire T, Roy YM, Tyrrell L. Non-prescription (OTC) oral analgesics for acute pain—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;11:CD010794.
Lichtenberger LM. Where is the evidence that cyclooxygenase inhibition is the primary cause of nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal injury? Topical injury revisited. Biochem Pharmacol. 2001;61(6):631–7.
Somasundaram S, Rafi S, Hayllar J, et al. Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut. 1997;41(3):344–53.
Somasundaram S, Hayllar H, Rafi S, Wrigglesworth JM, Macpherson AJ, Bjarnason I. The biochemical basis of non-steroidal anti-inflammatory drug-induced damage to the gastrointestinal tract: a review and a hypothesis. Scand J Gastroenterol. 1995;30(4):289–99.
Henry D, McGettigan P. Epidemiology overview of gastrointestinal and renal toxicity of NSAIDs. Int J Clin Pract Suppl. 2003;2003(135):43–9.
Garcia Rodriguez LA, Hernandez-Diaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res. 2001;2001(3):98–101.
Castellsague J, Riera-Guardia N, Calingaert B, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf. 2012;35(12):1127–46.
Bjarnason I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int J Clin Pract Suppl. 2013;178:37–42.
Blot WJ, McLaughlin JK. Over the counter non-steroidal anti-inflammatory drugs and risk of gastrointestinal bleeding. J Epidemiol Biostat. 2000;5(2):137–42.
Lewis JD, Kimmel SE, Localio AR, et al. Risk of serious upper gastrointestinal toxicity with over-the-counter nonaspirin nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005;129(6):1865–74.
Henry D, Lim LL, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ (Clin Res Ed). 1996;312(7046):1563–6.
Lewis SC, Langman MJ, Laporte JR, Matthews JN, Rawlins MD, Wiholm BE. Dose-response relationships between individual nonaspirin nonsteroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54(3):320–6.
Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63(7):759–66.
Masclee GM, Valkhoff VE, Coloma PM, et al. Risk of upper gastrointestinal bleeding from different drug combinations. Gastroenterology. 2014;147(4):784–792.e789 (quiz e713–784).
Henry D, McGettigan P. Epidemiology overview of gastrointestinal and renal toxicity of NSAIDs. Int J Clin Pract Suppl. 2003;135:43–9.
Doyle G, Furey S, Berlin R, et al. Gastrointestinal safety and tolerance of ibuprofen at maximum over-the-counter dose. Aliment Pharmacol Ther. 1999;13(7):897–906.
Fries JF, Bruce B. Rates of serious gastrointestinal events from low dose use of acetylsalicylic acid, acetaminophen, and ibuprofen in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2003;30(10):2226–33.
Le Parc JM, Van Ganse E, Moore N, Wall R, Schneid H, Verriere F. Comparative tolerability of paracetamol, aspirin and ibuprofen for short-term analgesia in patients with musculoskeletal conditions: results in 4291 patients. Clin Rheumatol. 2002;21(1):28–31.
Yeomans ND, Graham DY, Husni ME, et al. Randomised clinical trial: gastrointestinal events in arthritis patients treated with celecoxib, ibuprofen or naproxen in the PRECISION trial. Aliment Pharmacol Ther. 2018;47(11):1453–63.
Michels SL, Collins J, Reynolds MW, Abramsky S, Paredes-Diaz A, McCarberg B. Over-the-counter ibuprofen and risk of gastrointestinal bleeding complications: a systematic literature review. Curr Med Res Opin. 2012;28(1):89–99.
Biskupiak JE, Brixner DI, Howard K, Oderda GM. Gastrointestinal complications of over-the-counter nonsteroidal antiinflammatory drugs. J Pain Palliative Care Pharmacother. 2006;20(3):7–14.
Boureau F, Schneid H, Zeghari N, Wall R, Bourgeois P. The IPSO study: ibuprofen, paracetamol study in osteoarthritis. A randomised comparative clinical study comparing the efficacy and safety of ibuprofen and paracetamol analgesic treatment of osteoarthritis of the knee or hip. Ann Rheum Dis. 2004;63(9):1028–34.
Rafaniello C, Ferrajolo C, Sullo MG, et al. Risk of gastrointestinal complications associated to NSAIDs, low-dose aspirin and their combinations: results of a pharmacovigilance reporting system. Pharmacol Res. 2016;104:108–14.
van der Linden MW, van der Bij S, Welsing P, Kuipers EJ, Herings RM. The balance between severe cardiovascular and gastrointestinal events among users of selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2009;68(5):668–73.
Marco Garbayo JL, Koninckx Canada M, Perez Castello I, Faus Soler MT, Fuster Torres R, Moncho Escriva M. Cross-sectional analysis of retrospective case series of hospitalisations for gastropathy caused by non-steroidal anti-inflammatory treatment: risk factors and gastroprotection use. Eur J Hosp Pharm Sci Pract. 2017;24(6):355–60.
Bello AE, Kent JD, Holt RJ. Gastroprotective efficacy and safety of single-tablet ibuprofen/famotidine vs ibuprofen in older persons. Phys Sportsmed. 2015;43(3):193–9.
Bello AE, Kent JD, Grahn AY, Rice P, Holt RJ. Risk of upper gastrointestinal ulcers in patients with osteoarthritis receiving single-tablet ibuprofen/famotidine versus ibuprofen alone: pooled efficacy and safety analyses of two randomized, double-blind, comparison trials. Postgrad Med. 2014;126(4):82–91.
Bello AE, Grahn AY, Ball J, Kent JD, Holt RJ. One-year safety of ibuprofen/famotidine fixed combination versus ibuprofen alone: pooled analyses of two 24-week randomized, double-blind trials and a follow-on extension. Curr Med Res Opin. 2015;31(3):407–20.
Laine L, Kivitz AJ, Bello AE, Grahn AY, Schiff MH, Taha AS. Double-blind randomized trials of single-tablet ibuprofen/high-dose famotidine vs. ibuprofen alone for reduction of gastric and duodenal ulcers. Am J Gastroenterol. 2012;107(3):379–86.
Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet (Lond Engl). 1994;343(8900):769–72.
Hawkey CJ, Weinstein WM, Smalley W, et al. Effect of risk factors on complicated and uncomplicated ulcers in the TARGET lumiracoxib outcomes study. Gastroenterology. 2007;133(1):57–64.
Moore N, Salvo F, Duong M, Blin P, Pariente A. Cardiovascular risks associated with low-dose ibuprofen and diclofenac as used OTC. Expert Opin Drug Saf. 2014;13(2):167–79.
Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169(2):141–9.
Solomon DH, Avorn J, Sturmer T, Glynn RJ, Mogun H, Schneeweiss S. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006;54(5):1378–89.
Fosbol EL, Gislason GH, Jacobsen S, et al. Risk of myocardial infarction and death associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther. 2009;85(2):190–7.
Barcella CA, Lamberts M, McGettigan P, et al. Differences in cardiovascular safety with non-steroidal anti-inflammatory drug therapy—a nationwide study in patients with osteoarthritis. Basic Clin Pharmacol Toxicol. 2019;124(5):629–41.
Pepine CJ, Gurbel PA. Cardiovascular safety of NSAIDs: additional insights after PRECISION and point of view. Clin Cardiol. 2017;40(12):1352–6.
Farkouh ME, Verheugt FW, Ruland S, et al. A comparison of the blood pressure changes of lumiracoxib with those of ibuprofen and naproxen. J Clin Hypertens (Greenwich). 2008;10(8):592–602.
Ruschitzka F, Borer JS, Krum H, et al. Differential blood pressure effects of ibuprofen, naproxen, and celecoxib in patients with arthritis: the PRECISION-ABPM (prospective randomized evaluation of celecoxib integrated safety versus ibuprofen or naproxen ambulatory blood pressure measurement) trial. Eur Heart J. 2017;38(44):3282–92.
Gonzalez-Valcarcel J, Sissani L, Labreuche J, et al. Paracetamol, ibuprofen, and recurrent major cardiovascular and major bleeding events in 19,120 patients with recent ischemic stroke. Stroke. 2016;47(4):1045–52.
Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ (Clin Res Ed). 2011;342:c7086.
Lipworth L, Friis S, Blot WJ, et al. A population-based cohort study of mortality among users of ibuprofen in Denmark. Am J Ther. 2004;11(3):156–63.
Duong M, Abouelfath A, Lassalle R, Droz C, Blin P, Moore N. Coronary events after dispensing of ibuprofen: a propensity score-matched cohort study versus paracetamol in the French nationwide claims database sample. Drug Saf. 2018;41(11):1049–58.
Dong YH, Chang CH, Wu LC, Hwang JS, Toh S. Comparative cardiovascular safety of nonsteroidal anti-inflammatory drugs in patients with hypertension: a population-based cohort study. Br J Clin Pharmacol. 2018;84(5):1045–56.
Whelton A, Lefkowith JL, West CR, Verburg KM. Cardiorenal effects of celecoxib as compared with the nonsteroidal anti-inflammatory drugs diclofenac and ibuprofen. Kidney Int. 2006;70(8):1495–502.
Singh G, Wu O, Langhorne P, Madhok R. Risk of acute myocardial infarction with nonselective non-steroidal anti-inflammatory drugs: a meta-analysis. Arthritis Res Ther. 2006;8(5):R153.
Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation. 2011;123(20):2226–35.
Gislason GH, Jacobsen S, Rasmussen JN, et al. Risk of death or reinfarction associated with the use of selective cyclooxygenase-2 inhibitors and nonselective nonsteroidal antiinflammatory drugs after acute myocardial infarction. Circulation. 2006;113(25):2906–13.
Patel TN, Goldberg KC. Use of aspirin and ibuprofen compared with aspirin alone and the risk of myocardial infarction. Arch Intern Med. 2004;164(8):852–6.
Munoz Olmo L, Juan Armas J, Gomariz Garcia JJ. Risk of fatal/non-fatal events in patients with previous coronary heart disease/acute myocardial infarction and treatment with non-steroidal anti-inflammatory drugs. Semergen. 2018;44(5):355–63.
Huang WF, Hsiao FY, Wen YW, Tsai YW. Cardiovascular events associated with the use of four nonselective NSAIDs (etodolac, nabumetone, ibuprofen, or naproxen) versus a cyclooxygenase-2 inhibitor (celecoxib): a population-based analysis in Taiwanese adults. Clin Ther. 2006;28(11):1827–36.
Zingler G, Hermann B, Fischer T, Herdegen T. Cardiovascular adverse events by non-steroidal anti-inflammatory drugs: when the benefits outweigh the risks. Expert Rev Clin Pharmacol. 2016;9(11):1479–92.
Prozzi GR, Canas M, Urtasun MA, Buschiazzo HO, Dorati CM, Mordujovich-Buschiazzo P. Cardiovascular risk of non-steroidal anti-inflammatory drugs. Med (B Aires). 2018;78(5):349–55.
Schlondorff D. Renal compliations of nonsteroidal anti-inflammatory drugs. Kidney Internat. 1993;44:643–53.
Weir MR. Renal effects of nonselective NSAIDs and coxibs. Cleve Clin J Med. 2002;69(Suppl 1):Si53–8.
Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med. 1999;106:13S–24S.
American Geriatric Society, Panel. BCUE. American Geriatrics Society 2015 updated Beers croiteria for potentially inappropriate drug use in older adults. J Am Geriatr Soc. 2015;2015(63):2227–46.
Farquhar WB, Morgan AL, Zambraski EJ, Kenney WL. Effects of acetaminophen and ibuprofen on renal function in the stressed kidney. J Appl Physiol (1985). 1999;86(2):598–604.
Aweid O, Haider Z, Saed A, Kalairajah Y. Treatment modalities for hip and knee osteoarthritis: a systematic review of safety. J Orthopaedic Surg (Hong Kong). 2018;26(3):2309499018808669.
Griffin MR, Yared A, Ray WA. Nonsteroidal antiinflammatory drugs and acute renal failure in elderly persons. Am J Epidemiol. 2000;151(5):488–96.
Traversa G, Bianchi C, Da Cas R, Abraha I, Menniti-Ippolito F, Venegoni M. Cohort study of hepatotoxicity associated with nimesulide and other non-steroidal anti-inflammatory drugs. BMJ (Clin Res Ed). 2003;327(7405):18–22.
Bessone F. Non-steroidal anti-inflammatory drugs: what is the actual risk of liver damage? World J Gastroenterol. 2010;16(45):5651–61.
Rostom A, Goldkind L, Laine L. Nonsteroidal anti-inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clin Gastroenterol Hepatol. 2005;3(5):489–98.
Donati M, Conforti A, Lenti MC, et al. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case–control study in Italy. Br J Clin Pharmacol. 2016;82(1):238–48.
Gulmez SE, Unal US, Lassalle R, Chartier A, Grolleau A, Moore N. Risk of hospital admission for liver injury in users of NSAIDs and nonoverdose paracetamol: preliminary results from the EPIHAM study. Pharmacoepidemiol Drug Saf. 2018;27(11):1174–81.
Gulmez SE, Larrey D, Pageaux GP, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case-population SALT study. Drug Saf. 2013;36(2):135–44.
Pelucchi C, Grigoryan L, Galeone C, et al. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18(Suppl 1):1–28.
Lesko SM, O’Brien KL, Schwartz B, Vezina R, Mitchell AA. Invasive group A streptococcal infection and nonsteroidal antiinflammatory drug use among children with primary varicella. Pediatrics. 2001;107(5):1108–15.
Hamilton SM, Bayer CR, Stevens DL, Bryant AE. Effects of selective and nonselective nonsteroidal anti-inflammatory drugs on antibiotic efficacy of experimental group A streptococcal myonecrosis. J Infect Dis. 2014;209(9):1429–35.
Stevens DL. Could nonsteroidal antiinflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome? Clin Infect Dis. 1995;21(4):977–80.
Barnham MR, Weightman NC, Anderson AW, Tanna A. Streptococcal toxic shock syndrome: a description of 14 cases from North Yorkshire, UK. Clin Microbiol Infect. 2002;8(3):174–81.
Zerr DM, Alexander ER, Duchin JS, Koutsky LA, Rubens CE. A case–control study of necrotizing fasciitis during primary varicella. Pediatrics. 1999;103(4 Pt 1):783–90.
Aronoff DM, Bloch KC. Assessing the relationship between the use of nonsteroidal antiinflammatory drugs and necrotizing fasciitis caused by group A streptococcus. Medicine (Baltimore). 2003;82(4):225–35.
Lamagni TL, Neal S, Keshishian C, et al. Severe Streptococcus pyogenes infections, United Kingdom, 2003–2004. Emerg Infect Dis. 2008;14(2):202–9.
Moore N, Le Parc JM, van Ganse E, Wall R, Schneid H, Cairns R. Tolerability of ibuprofen, aspirin and paracetamol for the treatment of cold and flu symptoms and sore throat pain. Int J Clin Pract. 2002;56(10):732–4.
Eccles R. Efficacy and safety of over-the-counter analgesics in the treatment of common cold and flu. J Clin Pharm Ther. 2006;31(4):309–19.
Hamilton SM, Bayer CR, Stevens DL, Lieber RL, Bryant AE. Muscle injury, vimentin expression, and nonsteroidal anti-inflammatory drugs predispose to cryptic group A streptococcal necrotizing infection. J Infect Dis. 2008;198(11):1692–8.
Weng TC, Chen CC, Toh HS, Tang HJ. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J Microbiol Immunol Infect. 2011;44(6):418–23.
del Prado G, Martinez-Marin C, Huelves L, et al. Impact of ibuprofen therapy in the outcome of experimental pneumococcal acute otitis media treated with amoxicillin or erythromycin. Pediatr Res. 2006;60(5):555–9.
Lands LC, Stanojevic S. Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2013;6:CD001505.
Shah PN, Marshall-Batty KR, Smolen JA, et al. Antimicrobial activity of ibuprofen against cystic fibrosis-associated gram-negative pathogens. Antimicrob Agents Chemother. 2018;62:3.
Konstan MW. Ibuprofen therapy for cystic fibrosis lung disease: revisited. Curr Opin Pulm Med. 2008;14(6):567–73.
Kirkwood ZI, Millar BC, Downey DG, Moore JE. Antimycobacterial activity of nonantibiotics associated with the polypharmacy of cystic fibrosis (CF) against mycobacterium abscessus. Int J Mycobacteriol. 2018;7(4):358–60.
Sordelli DO, Cerquetti MC, el-Tawil G, Ramwell PW, Hooke AM, Bellanti JA. Ibuprofen modifies the inflammatory response of the murine lung to Pseudomonas aeruginosa. Eur J Respir Dis. 1985;67(2):118–27.
Kelley BP, Bennett KG, Chung KC, Kozlow JH. Ibuprofen may not increase bleeding risk in plastic surgery: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137(4):1309–16.
Dona I, Blanca-Lopez N, Torres MJ, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22(5):363–71.
Aun MV, Blanca M, Garro LS, et al. Nonsteroidal anti-inflammatory drugs are major causes of drug-induced anaphylaxis. J Allergy Clin Immunol Pract. 2014;2(4):414–20.
Torres MJ, Barrionuevo E, Kowalski M, Blanca M. Hypersensitivity reactions to nonsteroidal anti-inflammatory drugs. Immunol Allergy Clin North Am. 2014;34(3):507–24.
McMahon AD, Evans JMM, MacDonald TM. Hypersensitivity reactions associated with exposure to naproxen and ibuprofen: a cohort study. J Clin Epidemiol. 2001;54(12):1271–4.
Nanau RM, Neuman MG. Ibuprofen-induced hypersensitivity syndrome. Transl Res. 2010;155(6):275–93.
Kristensen DM, Desdoits-Lethimonier C, Mackey AL, et al. Ibuprofen alters human testicular physiology to produce a state of compensated hypogonadism. Proc Natl Acad Sci USA. 2018;115(4):E715–e724.
Ben Maamar M, Lesne L, Hennig K, et al. Ibuprofen results in alterations of human fetal testis development. Sci Rep. 2017;7:44184.
Akrami H, Moradi B, Borzabadi Farahani D, Mehdizadeh K. Ibuprofen reduces cell proliferation through inhibiting Wnt/beta catenin signaling pathway in gastric cancer stem cells. Cell Biol Int. 2018;42(8):949–58.
Akrami H, Aminzadeh S, Fallahi H. Inhibitory effect of ibuprofen on tumor survival and angiogenesis in gastric cancer cell. Tumour Biol. 2015;36(5):3237–43.
Dandah O, Najafzadeh M, Isreb M, et al. Aspirin and ibuprofen, in bulk and nanoforms: effects on DNA damage in peripheral lymphocytes from breast cancer patients and healthy individuals. Mutat Res Genet Toxicol Environ Mutagen. 2018;826:41–6.
Gay-Escoda C, Hanna M, Montero Matamala A, et al. Tramadol/dexketoprofen (TRAM/DKP) compared with tramadol/paracetamol in moderate to severe acute pain: results of a randomised, double-blind, placebo and active-controlled, parallel group trial in the impacted third molar extraction pain model (DAVID study). BMJ Open. 2019;9(2):e023715.
Paladini A, Fusco M, Coaccioli S, Skaper SD, Varrassi G. Chronic pain in the elderly: the case for new therapeutic strategies. Pain Physician. 2015;18(5):E863–76.
Acknowledgements
Funding
The research has been funded by the Paolo Procacci Foundation with an unconditional grant. The Rapid Service Fee was funded by Abbott Established Pharmaceuticals Division (EPD).
Medical Writing and/or Editorial Assistance
The authors are grateful to Jo Ann LeQuang of LeQ Medical for her support in writing and editing of the paper. This support was funded by Abbott Established Pharmaceuticals Division (EPD). The authors also thank Narimane Benhassine for her review.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors participated in the design of the review, search and analysis of the background literature and have reviewed and approved the final version of the manuscript.
Disclosures
Giustino Varrassi served as consultant for Abbott, Dompé Farmaceutici, Malesci, Menarini, Molteni, Mundipharma, Shionogi, and Takeda and is a member of the journal’s Editorial Board. Joseph V. Pergolizzi has been consultant for BDSI, Daiichi, Dompé Farmaceutici, Enalare, Grünenthal, Hikma, Neumentum, Salix, and US World MEDS and is a member of the journal’s Editorial Board. Antonella Paladini served as speaker for Molteni Farmaceutici and is a member of the journal’s Editorial Board. Pascal Dowling is an employee of Abbott.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10075727.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Varrassi, G., Pergolizzi, J.V., Dowling, P. et al. Ibuprofen Safety at the Golden Anniversary: Are all NSAIDs the Same? A Narrative Review. Adv Ther 37, 61–82 (2020). https://doi.org/10.1007/s12325-019-01144-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01144-9