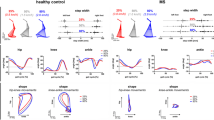
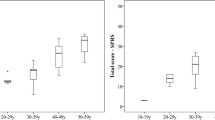
Abstract
Mobility limitations, including a decrease in walking speed, are major issues for people with autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). Improving our understanding of factors influencing walking speed in ARSACS may inform the development of future interventions for gait rehabilitation and contribute to better clinical practices. The objective of the study was to identify the factors influencing the self-selected walking speed in adults with ARSACS. The dependent variable of this cross-sectional study was the self-selected speed and the factors (independent variables) were age, sex, balance, balance confidence, knee flexion and extension cocontraction indexes, lower limb coordination, passive range of motion of ankle dorsiflexion, knee and hip extension, and global spasticity. Multiple regression models were used to assess the relationships between walking speed and each factor individually. Six factors were significantly associated with walking speed and thus included in regression models. The models explained between 42.4 and 66.5% of the total variance of the self-selected walking speed. The factors that most influence self-selected walking speed are balance and lower limb coordination. In order of importance, the other factors that also significantly influence self-selected walking speed are ankle dorsiflexion range of motion, lower limb spasticity, knee extension range of motion, and confidence in balance. Balance and lower limb coordination should be targeted in rehabilitation interventions to maintain walking ability and functional independence as long as possible. The six factors identified should also be included in future studies to deepen our understanding of walking speed.

Similar content being viewed by others
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator, following approval of the data use proposal by the Ethics Review Board of the CIUSSS-SLSJ.
Abbreviations
- 10mWT:
-
10-m walk test
- ABC-simplified:
-
Activity-specific balance confidence—simplified
- ARSACS:
-
Autosomal recessive spastic ataxia of Charlevoix-Saguenay
- BBS:
-
Berg balance scale
- CI:
-
Cocontraction index
- EMG:
-
Electromyography
- ICC:
-
Intraclass correlation coefficient
- LEMOCOT:
-
Lower extremity motor coordination test
- MAS:
-
Modified Ashworth scale
- SD:
-
Standard deviation
References
Bouchard JP, Barbeau A, Bouchard R, Bouchard RW. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Can J Neurol Sci. 1978;5:61–9.
Vermeer S, van de Warrenburg BP, Kamsteeg E-J, et al. Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong C-T, Smith RJH, Stephens K, editors. ARSACS. GeneRewiews [Internet]. Seattle (WA), University of Washinton. 2003 Dec 9 [Updated 2020 Jan 2]. pp. http://www.ncbi.nlm.nih.gov/books/NBK1255/
Bouchard JP, Richter A, Mathieu J, Brunet D, Hudson TJ, Morgan K, Melançon SB. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Neuromuscul Disord. 1998;8:474–9.
Gagnon C, Brais B, Lessard I, Lavoie C, Côté I, Mathieu J. From motor performance to participation: a quantitative descriptive study in adults with autosomal recessive spastic ataxia of Charlevoix-Saguenay. Orphanet J Rare Dis. 2018;13:165.
Lessard I, Brais B, Cote I, Lavoie C, Synofzik M, Mathieu J, Gagnon C. Assessing mobility and balance in autosomal recessive spastic ataxia of Charlevoix-Saguenay population: Validity and reliability of four outcome measures. J Neurol Sci. 2018;390:4–9. https://doi.org/10.1016/j.jns.2018.03.033.
Klimpe S, Schule R, Kassubek J, Otto S, Kohl Z, Klebe S, Klopstock T, Ratzka S, Karle K, Schols L. Disease severity affects quality of life of hereditary spastic paraplegia patients. Eur J Neurol. 2012;19:168–71. https://doi.org/10.1111/j.1468-1331.2011.03443.x.
Schniepp R, Schlick C, Pradhan C, Dieterich M, Brandt T, Jahn K, Wuehr M. The interrelationship between disease severity, dynamic stability, and falls in cerebellar ataxia. J Neurol. 2016;263:1409–17. https://doi.org/10.1007/s00415-016-8142-z.
Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32:46–9.
Morton SM, Bastian AJ. Relative contributions of balance and voluntary leg-coordination deficits to cerebellar gait ataxia. J Neurophysiol. 2003;89:1844–56. https://doi.org/10.1152/jn.00787.2002.
Lessard I, Lavoie C, Cote I, Mathieu J, Brais B, Gagnon C. Validity and reliability of the LEMOCOT in the adult ARSACS population: a measure of lower limb coordination. J Neurol Sci. 2017;377:193–6. https://doi.org/10.1016/j.jns.2017.03.046.
Lavoie C, Mathieu J, Gagnon C. Développement et validation de l’échelle de gravité de l’ataxie récessive spastique de Charlevoix-Saguenay (DSI-ARSACS) : section pyramidale. Université de Sherbrooke; 2015.
Krygier M, Konkel A, Schinwelski M, Rydzanicz M, Walczak A, Sildatke-Bauer M, Ploski R, Slawek J. Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) - a Polish family with novel SACS mutations. Neurol Neurochir Pol. 2017; https://doi.org/10.1016/j.pjnns.2017.08.003.
Gagnon C, Lessard I, Lavoie C, Cote I, St-Gelais R, Mathieu J, Brais B. An exploratory natural history of ataxia of Charlevoix-Saguenay: a 2-year follow-up. Neurology. 2018; https://doi.org/10.1212/wnl.0000000000006290.
Lessard I, Côté I, St-Gelais R, Hébert LJ, Brais B, Mathieu J, Rodrigue X, Gagnon C. Natural History of Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay: a 4-Year Longitudinal Study. Cerebellum. 2023. https://doi.org/10.1007/s12311-023-01558-w.
Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Int Disabil Stud. 1990;12:6–9.
Berg K, Wood-Dauphinee S, Williams JI, Gayton D. Measuring balance in elderly : preliminary development of an instrument. Physiother Can. 1989;41:304–11.
Filiatrault J, Gauvin L, Fournier M, Parisien M, Robitaille Y, Laforest S, Corriveau H, Richard L. Evidence of the psychometric qualities of a simplified version of the activities-specific balance confidence scale for community-dwelling seniors. Arch Phys Med Rehabil. 2007;88:664–72. https://doi.org/10.1016/j.apmr.2007.02.003.
Busse ME, Wiles CM, van Deursen RWM. Muscle co-activation in neurological conditions. Phys Ther Rev. 2005;10:247–53. https://doi.org/10.1179/108331905X78915.
Desrosiers J, Rochette A, Corriveau H. Validation of a new lower-extremity motor coordination test. Arch Phys Med Rehabil. 2005;86:993–8. S0003999304013863 [pii]. https://doi.org/10.1016/j.apmr.2004.11.007.
Clarkson HM. Musculoskeletal assessment: joint motion and muscle testing. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012.
Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–7.
Bohannon RW. Population representative gait speed and its determinants. J Geriatr Phys Ther. 2008;31:49–52.
Christofoletti G, McNeely ME, Campbell MC, Duncan RP, Earhart GM. Investigation of factors impacting mobility and gait in Parkinson disease. Hum Mov Sci. 2016;49:308–14. https://doi.org/10.1016/j.humov.2016.08.007.
Lee KB, Lim SH, Kim YD, Yang BI, Kim KH, Lee KS, Kim EJ, Hwang BY. The contributions of balance to gait capacity and motor function in chronic stroke. J Phys Ther Sci. 2016;28:1686–90. https://doi.org/10.1589/jpts.28.1686.
Pilliod J, Moutton S, Lavie J, Maurat E, Hubert C, Bellance N, Anheim M, Forlani S, Mochel F, N'Guyen K, Thauvin-Robinet C, Verny C, Milea D, Lesca G, Koenig M, Rodriguez D, Houcinat N, Van-Gils J, Durand CM, et al. New practical definitions for the diagnosis of autosomal recessive spastic ataxia of Charlevoix-Saguenay. Ann Neurol. 2015. https://doi.org/10.1002/ana.24509.
Kinsella S, Moran K. Gait pattern categorization of stroke participants with equinus deformity of the foot. Gait Posture. 2008;27:144–51. https://doi.org/10.1016/j.gaitpost.2007.03.008.
Braschinsky M, Parts K, Maamagi H, Gross-Paju K, Haldre S. Functional assessment of lower extremities in hereditary spastic paraplegia. Arch Phys Med Rehabil. 2009;90:1887–90. https://doi.org/10.1016/j.apmr.2009.06.016.
Rousseaux M, Launay MJ, Kozlowski O, Daveluy W. Botulinum toxin injection in patients with hereditary spastic paraparesis. Eur J Neurol. 2007;14:206–12. https://doi.org/10.1111/j.1468-1331.2006.01617.x.
Klebe S, Stolze H, Kopper F, Lorenz D, Wenzelburger R, Volkmann J, Porschke H, Deuschl G. Gait analysis of sporadic and hereditary spastic paraplegia. J Neurol. 2004;251:571–8. https://doi.org/10.1007/s00415-004-0366-7.
Richards C, Bouchard JP, Bouchard R, Barbeau H. A preliminary study of dynamic muscle function in hereditary ataxia. Can J Neurol Sci. 1980;7:367–77.
Foongsathaporn C, Panyakaew P, Jitkritsadakul O, Bhidayasiri R. What daily activities increase the risk of falling in Parkinson patients? An analysis of the utility of the ABC-16 scale. J Neurol Sci. 2016;364:183–7. https://doi.org/10.1016/j.jns.2016.03.037.
Torkia C, Best KL, Miller WC, Eng JJ. Balance confidence: a predictor of perceived physical function, perceived mobility, and perceived recovery 1 year after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2016;97:1064–71. https://doi.org/10.1016/j.apmr.2016.03.004.
Acknowledgements
The authors would like to thank all patients who participated in this study as their involvement is essential to the success of the research. The authors would like to thank Djamal Berbiche for his support in the statistical analysis.
Funding
This study was supported by the European Union’s Horizon 2020 research and innovation program under the ERA-NET Cofund action no. 643578. It was also supported by the CIFR team (MDC and Ataxia of Charlevoix-Saguenay) and Ataxia of Charlevoix-Saguenay foundation (to C.G. and B.B.) and the BMBF (01GM1607), under the frame of the E-Rare-3 network PREPARE. Funding sources also include the Canadian Institutes of Health Research in partnership with the Ataxia of Charlevoix-Saguenay Foundation (Emerging Team Grant no TR2-119189). CG holds a career-grant funding from Fonds de recherche en santé du Québec (no 31011). Sponsors did not contribute to the design, other study steps, or data analysis.
Author information
Authors and Affiliations
Contributions
IL, BB, JM, and CG contributed to the study concept and design. IL and RSG had a major role in the data acquisition. IL, IC, LJH, BB, and CG contributed to data analysis and interpretation. The first draft of the manuscript was written by IL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Ethics Review Board of the Centre intégré universitaire de santé et de services sociaux du Saguenay–Lac-Saint-Jean (#2015-002) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to Participate
Written informed consent was obtained for all participants enrolled in the study.
Competing Interest
C.G. is a consultant for Vertex, Arthrex, Seelos, and Biogen, and also conducts academic work for Biogen, Ionis, and Seelos, all unrelated to the present manuscript. All other authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lessard, I., Hébert, L.J., St-Gelais, R. et al. Toward a Better Understanding of Walking Speed in Ataxia of Charlevoix-Saguenay: a Factor Exploratory Study. Cerebellum (2023). https://doi.org/10.1007/s12311-023-01646-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s12311-023-01646-x