Abstract
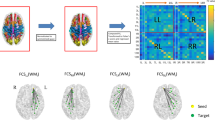
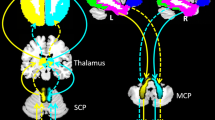
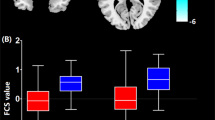
We aimed to explore the altered functional connectivity patterns within cerebello-cerebral circuits in temporal lobe epilepsy (TLE) patients with and without focal to bilateral tonic–clonic seizures (FBTCS). Forty-two patients with unilateral TLE (21 with and 21 without FBTCS) and 22 healthy controls were recruited. We chose deep cerebellar nuclei as seed regions, calculated static and dynamic functional connectivity (sFC and dFC) in the patients with and without FBTCS and healthy controls, and compared sFC and dFC among the three groups. Correlation analyses were used to assess relationships between the significantly altered imaging features and patient clinical parameters. Compared to the group without FBTCS, the FBTCS group showed decreased sFC between the right dentate nuclei and left hemisphere regions including the middle frontal gyrus, superior temporal gyrus, superior medial frontal gyrus and posterior cingulate gyrus, and significantly increased dFC between the right interposed nuclei and contralateral precuneus. Relative to HCs, the FBTCS group demonstrated prominently decreased sFC between the right dentate nuclei and left middle frontal gyrus. No significant correlations between the altered imaging features and patient clinical parameters were observed. Our results suggest that the disrupted cerebello-cerebral FC might be related to cognitive impairment, epileptogenesis, and propagation of epileptic activities in patients with FBTCS.



Similar content being viewed by others
References
Lim SN, Lee CY, Lee ST, Tu PH, Chang BL, Lee CH, et al. Low and high frequency hippocampal stimulation for drug-resistant mesial temporal lobe epilepsy. Neuromodulation. 2016;19:365–72. https://doi.org/10.1111/ner.12435.
Caciagli L, Bernasconi A, Wiebe S, Koepp MJ, Bernasconi N, Bernhardt BC. A meta-analysis on progressive atrophy in intractable temporal lobe epilepsy: time is brain? Neurology. 2017;89:506–16. https://doi.org/10.1212/WNL.0000000000004176.
Keller SS, Richardson MP, Schoene-Bake JC, O’Muircheartaigh J, Elkommos S, Kreilkamp B, et al. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol. 2015;77:760–74. https://doi.org/10.1002/ana.24376.
Zangiabadi N, Ladino LD, Sina F, Orozco-Hernández JP, Carter A, Téllez-Zenteno JF. Deep brain stimulation and drug-resistant epilepsy: a review of the literature. Front Neurol. 2019;10:1–18. https://doi.org/10.3389/fneur.2019.006012019.00601.
Krauss GL, Koubeissi MZ. Cerebellar and thalamic stimulation treatment for epilepsy. Acta Neurochir Suppl. 2007; 97(97 PART 2):347–56.
Streng ML, Krook-Magnuson E. Excitation, but not inhibition, of the fastigial nucleus provides powerful control over temporal lobe seizures. vol. 598. 2020. https://doi.org/10.1113/JP278747.
Jiang S, Li X, Li Z, Chang X, Chen Y, Huang Y, et al. Cerebello-cerebral connectivity in idiopathic generalized epilepsy. EurRadiol. 2020;30:3924–33. https://doi.org/10.1007/s00330-020-06674-3.
Zhang XY, Wang JJ, Zhu JN. Cerebellar fastigial nucleus: from anatomic construction to physiological functions. Cerebellum and Ataxias. 20165;3:1–10. https://doi.org/10.1186/s40673-016-0047-1.
Krauss GL, Koubeissi MZ. Cerebellar and thalamic stimulation treatment for epilepsy. Acta Neurochir Suppl. 2007;97:347–56. https://doi.org/10.1007/978-3-211-33081-4_40.
Rubio C, Custodio V, González E, Retana-Márquez S, López M, Paz C. Effects of kainic acid lesions of the cerebellar interpositus and dentate nuclei on amygdaloid kindling in rats. Brain Res Bull. 2011;85:64–7. https://doi.org/10.1016/j.brainresbull.2011.02.003.
Rijkers K, Moers-Hornikx VMP, Hemmes RJ, Aalbers MW, Temel Y, Vles JSH, et al. Sustained reduction of cerebellar activity in experimental epilepsy. Biomed Res Int 2015;2015. https://doi.org/10.1155/2015/718591.
Cooper IS, Upton ARM. Therapeutic implications of modulation of metabolism and functional activity of cerebral cortex by chronic stimulation of cerebellum and thalamus. Biol Psychiatry. 1985;20:811–3. https://doi.org/10.1016/0006-3223(85)90164-7.
Streng ML, Krook-Magnuson E. The cerebellum and epilepsy. Epilepsy Behav 2020:106909. https://doi.org/10.1016/j.yebeh.2020.106909.
Zhou X, Zhang Z, Liu J, Qin L, Pang X, Zheng J. Disruption and lateralization of cerebellar–cerebral functional networks in right temporal lobe epilepsy: a resting-state fMRI study. Epilepsy Behav. 2019;96:80–6. https://doi.org/10.1016/j.yebeh.2019.03.020.
Dumkrieger G, Chong CD, Ross K, Berisha V, Schwedt TJ. Static and dynamic functional connectivity differences between migraine and persistent post-traumatic headache: a resting-state magnetic resonance imaging study. Cephalalgia. 2019;39:1366–81. https://doi.org/10.1177/0333102419847728.
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE Official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. https://doi.org/10.1111/epi.12550.
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21. https://doi.org/10.1111/epi.13709.
Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30. https://doi.org/10.1111/epi.13670.
Yang L, Li H, Zhu L, Yu X, Jin B, Chen C, et al. Localized shape abnormalities in the thalamus and pallidum are associated with secondarily generalized seizures in mesial temporal lobe epilepsy. Epilepsy Behav. 2017;70:259–64. https://doi.org/10.1016/j.yebeh.2017.02.011.
Diedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage. 2011;54:1786–94. https://doi.org/10.1016/j.neuroimage.2010.10.035.
Yan CG, Di WX, Zuo XN, Zang YF. DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–51. https://doi.org/10.1007/s12021-016-9299-4.
Fu Z, Caprihan A, Chen J, Du Y, Adair JC, Sui J, et al. Altered static and dynamic functional network connectivity in Alzheimer’s disease and subcortical ischemic vascular disease: shared and specific brain connectivity abnormalities. Hum Brain Mapp. 2019;40:3203–21. https://doi.org/10.1002/hbm.24591.
Olivito G, Serra L, Marra C, Di Domenico C, Caltagirone C, Toniolo S, et al. Cerebellar dentate nucleus functional connectivity with cerebral cortex in Alzheimer’s disease and memory: a seed-based approach. Neurobiol Aging. 2020;89:32–40. https://doi.org/10.1016/j.neurobiolaging.2019.10.026.
Pang Y, Zhang H, Cui Q, Yang Q, Lu F, Chen H, et al. Combined static and dynamic functional connectivity signatures differentiating bipolar depression from major depressive disorder. Aust N Z J Psychiatry. 2020;54:832–42. https://doi.org/10.1177/0004867420924089.
Zhang W, Li S, Wang X, Gong Y, Yao L, Xiao Y, et al. Abnormal dynamic functional connectivity between speech and auditory areas in schizophrenia patients with auditory hallucinations. NeuroImage Clin. 2018;19:918–24. https://doi.org/10.1016/j.nicl.2018.06.018.
Marcián V, Mareček R, Pail M, Brázdil M. Cerebrocerebellar structural covariance in temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Behav 2020;111.https://doi.org/10.1016/j.yebeh.2020.107180.
Takahashi M, Shinoda Y. Neural circuits of inputs and outputs of the cerebellar cortex and nuclei. Neuroscience. 2020. https://doi.org/10.1016/j.neuroscience.2020.07.051.
Küper M, Thürling M, Maderwald S, Ladd ME, Timmann D. Structural and functional magnetic resonance imaging of the human cerebellar nuclei. Cerebellum. 2012;11:314–24. https://doi.org/10.1007/s12311-010-0194-5.
Habas C. Functional imaging of the deep cerebellar nuclei: a review. Cerebellum. 2010;9:22–8. https://doi.org/10.1007/s12311-009-0119-3.
Habas C, Manto M, Cabaraux P. The cerebellar thalamus. Cerebellum. 2019;18:635–48. https://doi.org/10.1007/s12311-019-01019-3.
Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Default mode network abnormalities in mesial temporal lobe epilepsy: a study combining fMRI and DTI. Hum Brain Mapp. 2011;32:883–95. https://doi.org/10.1002/hbm.21076.
Ofer I, LeRose C, Mast H, LeVan P, Metternich B, Egger K, et al. Association between seizure freedom and default mode network reorganization in patients with unilateral temporal lobe epilepsy. Epilepsy Behav. 2019;90:238–46. https://doi.org/10.1016/j.yebeh.2018.10.025.
Liu J, Zhang Z, Zhou X, Pang X, Liang X, Huang H, et al. Disrupted alertness and related functional connectivity in patients with focal impaired awareness seizures in temporal lobe epilepsy. Epilepsy Behav 2020;112. https://doi.org/10.1016/j.yebeh.2020.107369.
Horvath A, Kiss M, Szucs A, Kamondi A. Precuneus-dominant degeneration of parietal lobe is at risk of epilepsy in mild Alzheimer’s disease. Front Neurol. 2019;10:1–9. https://doi.org/10.3389/fneur.2019.00878.
Mohan A, Roberto AJ, Mohan A, Lorenzo A, Jones K, Carney MJ, et al. The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: a review. vol. 89. 2016.
Ke M, Jin B, Liu G, Yang X. Impairments of cingulated cortex in the generalized tonic-clonic seizure epilepsy by combining morphological and functional connectivity magnetic resonance imaging. J Integr Neurosci. 2017;16:429–39. https://doi.org/10.3233/JIN-170026.
Kros L, EelkmanRooda OHJ, Spanke JK, Alva P, Van Dongen MN, Karapatis A, et al. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann Neurol. 2015;77:1027–49. https://doi.org/10.1002/ana.24399.
Song P, Lin H, Liu C, Jiang Y, Lin Y, Xue Q, et al. Transcranial magnetic stimulation to the middle frontal gyrus during attention modes induced dynamic module reconfiguration in brain networks. Front Neuroinform. 2019;13:1–9. https://doi.org/10.3389/fninf.2019.00022.
Lu X, Miyachi S, Takada M. Anatomical evidence for the involvement of medial cerebellar output from the interpositus nuclei in cognitive functions. Proc Natl Acad Sci U S A. 2012;109:18980–4. https://doi.org/10.1073/pnas.1211168109.
Thürling M, Kahl F, Maderwald S, Stefanescu RM, Schlamann M, Boele HJ, et al. Cerebellar cortex and cerebellar nuclei are concomitantly activated during eyeblink conditioning: a 7T fMRI study in humans. J Neurosci. 2015;35:1228–39. https://doi.org/10.1523/JNEUROSCI.2492-14.2015.
Cunningham SI, Tomasi D, Volkow ND. Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum Brain Mapp. 2017;38:938–56. https://doi.org/10.1002/hbm.23429.
Koch G, Bonnì S, Pellicciari MC, Casula EP, Mancini M, Esposito R, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169:302–11. https://doi.org/10.1016/j.neuroimage.2017.12.048.
Acknowledgements
We would like to thank Professor Wei Ye for his contribution to the imaging data collection and all patients for their participation in the study. The authors also would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Funding
The research funding for this subject was provided by the National Natural Science Foundation of China (81560223) and by the Natural Science Foundation of Guangxi Province (2018GXNSFAA050149).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We confirm that we have read the journal’s position on issues related to ethical publication and affirm that this report is consistent with those guidelines.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nie, L., Jiang, Y., Lv, Z. et al. Deep Cerebellar Nuclei Functional Connectivity with Cerebral Cortex in Temporal Lobe Epilepsy With and Without Focal to Bilateral Tonic–Clonic Seizures: a Resting-State fMRI Study. Cerebellum 21, 253–263 (2022). https://doi.org/10.1007/s12311-021-01266-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-021-01266-3