Abstract
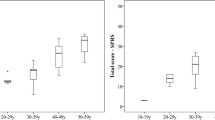
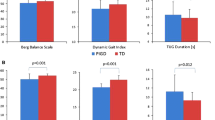
Friedreich’s ataxia (FRDA) is a rare autosomal-recessive slowly progressive neurodegenerative disorder. As common clinical measures for this devastating disease lack sensitivity, we explored whether (a) the quantitative motor assessments of the Q-Motor battery can enhance clinical characterisation of FRDA; (b) clinical measures can predict Q-Motor outcomes and (c) Q-Motor is sensitive to longitudinal change. At baseline 29 patients and 23 controls and in a 1-year follow-up 14 patients and 6 controls were included. The Q-Motor included lift (manumotography), finger tapping (digitomotography) and pronate/supinate (dysdiadochomotography) tasks. To model responses, a search of generalised linear models was conducted, selecting best fitting models, using demographic and clinical data as predictors. Predictors from selected models were used in linear mixed models to investigate longitudinal changes. Patients with FRDA performed worse than controls on most measures. Modelling of the pronate/supinate task was dominated by SCAFI (SCA functional index) subtasks, while tapping task and lift task models suggested a complex relationship with clinical measures. Longitudinal modelling implied minor changes from baseline to follow-up, while clinical scales mainly showed no change in this sample. Overall Q-Motor likely has favourable properties for assessing distinct motor aspects in severe FRDA as it can be administered in wheelchair-bound patients. Further longitudinal research is warranted to fully characterise its relation to routinely used measures and scales for FRDA.




Similar content being viewed by others
References
Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science (80- ). 1996;271(5254):1423–7. Available from: https://doi.org/10.1126/science.271.5254.1423
Vankan P. Prevalence gradients of Friedreich’s ataxia and R1b haplotype in Europe co-localize, suggesting a common Palaeolithic origin in the Franco-Cantabrian ice age refuge. J Neurochem. 2013;126(SUPPL.1):11–20.
Reetz K, Dogan I, Hohenfeld C, Didszun C, Giunti P, Mariotti C, et al. Nonataxia symptoms in Friedreich ataxia. Neurology. 2018 Aug 10; https://doi.org/10.1212/WNL.0000000000006121.
Bürk K. Friedreich ataxia: current status and future prospects. Cerebellum & Ataxias. 2017;4(1):4. Available from: https://doi.org/10.1186/s40673-017-0062-x
Delatycki MB, Corben LA. Clinical features of Friedreich ataxia. J Child Neurol 2012;27(9):1133–1137. Available from: https://doi.org/10.1177/0883073812448230
Reetz K, Dogan I, Costa AS, Dafotakis M, Fedosov K, Giunti P, et al. Biological and clinical characteristics of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS) cohort: a cross-sectional analysis of baseline data. Lancet Neurol. 2015;14(2):174–82.
Reetz K, Dogan I, Hilgers RD, Giunti P, Mariotti C, Durr A, et al. Progression characteristics of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS): a 2 year cohort study. Lancet Neurol. 2016;15(13):1346–54.
Schmitz-Hübsch T, Du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia. Neurol Int. 2006;66(1):1717–20.
Schmitz-Hubsch T, Giunti P, Stephenson DA, Globas C, Baliko L, Sacca F, Mariotti C, Rakowicz M, Szymanski S, Infante J, van de Warrenburg BPC, Timmann D, Fancellu R, Rola R, Depondt C, Schols L, Zdzienicka E, Kang J S, Dohlinger S, Kremer B, Melegh B, Filla A, Klockgether T SCA Functional Index: a useful compound performance measure for spinocerebellar ataxia. Neurol Int 2008 Aug 12;71(7):486–492. Available from: https://doi.org/10.1212/01.wnl.0000324863.76290.19
Jacobi H, Rakowicz M, Rola R, Fancellu R, Mariotti C, Charles P, et al. Inventory of non-ataxia signs (INAS): validation of a new clinical assessment instrument. Cerebellum. 2013;12(3):418–28.
Du Montcel ST, Charles P, Ribai P, Goizet C, Le Bayon A, Labauge P, et al. Composite cerebellar functional severity score: validation of a quantitative score of cerebellar impairment. Brain. 2008;131(5):1352–61.
Tanguy Melac A, Mariotti C, Filipovic Pierucci A, Giunti P, Arpa J, Boesch S, et al. Friedreich and dominant ataxias: quantitative differences in cerebellar dysfunction measurements. J Neurol Neurosurg Psychiatry. 2017;jnnp-2017-316964. Available from: https://doi.org/10.1136/jnnp-2017-316964%0A
Corben LA, Tai G, Wilson C, Collins V, Churchyard AJ, Delatycki MB. A comparison of three measures of upper limb function in Friedreich ataxia. J Neurol 2010 Apr 13;257(4):518–523. Available from: https://doi.org/10.1007/s00415-009-5352-7
Pandolfo M. Friedreich ataxia: the clinical picture. J Neurol. 2009;256(SUPPL. 1):3–8.
Gordon A M, Quinn L, Reilmann R, Marder K. Coordination of prehensile forces during precision grip in Huntington’s disease. Exp Neurol. 2000;163(1):136–48.
Reilmann R, Kirsten F, Quinn L, Henningsen H, Marder K, Gordon AM. Objective assessment of progression in Huntington’s disease: a 3-year follow-up study. Neurol Int 2001 Sep 11;57(5):920–924. Available from: https://doi.org/10.1212/WNL.57.5.920
Reilmann R, Schubert R. Motor outcome measures in Huntington disease clinical trials. In: Handbook of Clinical Neurology. 1st ed. Elsevier B.V.; 2017. p. 209–25. Available from: https://doi.org/10.1016/B978-0-12-801893-4.00018-3
Reilmann R, Holtbernd F, Bachmann R, Mohammadi S, Ringelstein EB, Deppe M. Grasping multiple sclerosis: do quantitative motor assessments provide a link between structure and function? J Neurol. 2013;260(2):407–14.
Holtbernd F, Deppe M, Bachmann R, Mohammadi S, Ringelstein EB, Reilmann R. Deficits in tongue motor control are linked to microstructural brain damage in multiple sclerosis: a pilot study. BMC Neurol. 2015 Dec 8;15(1):190. Available from: https://doi.org/10.1186/s12883-015-0451-9
Maetzler W, Ellerbrock M, Heger T, Sass C, Berg D, Reilmann R. Digitomotography in Parkinson’s disease: a cross-sectional and longitudinal study. Siegel A, editor. PLoS One. 2015 Apr 22;10(4):e0123914. Available from: https://doi.org/10.1371/journal.pone.0123914
Schaeffer E, Maetzler W, Liepelt-Scarfone I, Sass C, Reilmann R, Berg D. Quantitative motor assessment of dyskinesias in Parkinson’s disease. J Neural Transm 2015;122(9):1271–1278. Available from: https://doi.org/10.1007/s00702-015-1383-7
Hoffmann S, Siedler J, Brandt AU, Piper SK, Kohler S, Sass C, et al. Quantitative motor assessment of muscular weakness in myasthenia gravis: a pilot study. BMC Neurol. 2015;1–7. Available from: https://doi.org/10.1186/s12883-015-0517-8
Jeppesen Kragh F, Bruun N, Budtz-Jørgensen E, Hjermind LE, Schubert R, Reilmann R, et al. Quantitative measurements of motor function in Alzheimer’s disease, frontotemporal dementia, and dementia with Lewy bodies: a proof-of-concept study. Dement Geriatr Cogn Disord. 2018;46:168–79.
Hashimoto Y, Honda T, Matsumura K, Nakao M, Soga K, Katano K, et al. Quantitative evaluation of human cerebellum-dependent motor learning through prism adaptation of hand-reaching movement. PLoS One. 2015;10(3):1–19.
Lee J, Kagamihara Y, Kakei S. A new method for functional evaluation of motor commands in patients with cerebellar ataxia. PLoS One. 2015;10(7):1–22.
Menegoni F, Milano E, Trotti C, Galli M, Bigoni M, Baudo S, et al. Quantitative evaluation of functional limitation of upper limb movements in subjects affected by ataxia. Eur J Neurol. 2009;16(2):232–9.
Sanguineti V, Morasso PG, Baratto L, Brichetto G, Mancardi GL, Solaro C. Cerebellar ataxia: quantitative assessment and cybernetic interpretation. Hum Mov Sci. 2003;22(2):189–205.
Klein A, Sacrey LAR, Dunnett SB, Whishaw IQ, Nikkhah G. Proximal movements compensate for distal forelimb movement impairments in a reach-to-eat task in Huntington’s disease: new insights into motor impairments in a real-world skill. Neurobiol Dis 2011;41(2):560–569. Available from: https://doi.org/10.1016/j.nbd.2010.11.002
Giancardo L, Sánchez-Ferro A, Arroyo-Gallego T, Butterworth I, Mendoza CS, Montero P, Matarazzo M, Obeso JA, Gray ML, Estépar RSJ Computer keyboard interaction as an indicator of early Parkinson’s disease. Sci Rep 2016;6:1–10. Available from: https://doi.org/10.1038/srep34468
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar [cited 2015 Apr 20];9(1):97–113.
World Medical Association. World Medical Association Declaration of Helsinki. JAMA. 2013 Nov 27;310(20):2191. Available from: https://doi.org/10.1001/jama.2013.281053
Bechtel N, Scahill RI, Rosas HD, Acharya T, van den Bogaard SJA, Jauffret C, Say MJ, Sturrock A, Johnson H, Onorato CE, Salat DH, Durr A, Leavitt BR, Roos RAC, Landwehrmeyer GB, Langbehn DR, Stout JC, Tabrizi SJ, Reilmann R Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurol Int 2010 Dec 14;75(24):2150–2160. Available from: https://doi.org/10.1212/WNL.0b013e3182020123
Reilmann R, Bohlen S, Klopstock T, Bender A, Weindl A, Saemann P, Auer DP, Ringelstein EB, Lange HW Grasping premanifest Huntington’s disease—shaping new endpoints for new trials. Mov Disord 2010 Dec 15;25(16):2858–2862. Available from: https://doi.org/10.1002/mds.23300
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
Hurvich CM, Tsai C-L. Regression and time series model selection in small samples. Biometrika. 1989 Jun;76(2):297.
Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–23.
Calcagno V, Mazancourt C De. glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Softw 2010;34(12):1–29.
Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection 2 methods for accuracy estimation. Proc of IJCAI’95. 1995;1137–45.
Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bull. 1946 Dec;2(6):110. Available from: https://doi.org/10.1002/9780470057339.vai016
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015 Jun 23;67(1):883–5.
Nakagawa S, Johnson PCD, Schielzeth H. The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface. 2017 Sep;14(134):20170213. Available from: https://doi.org/10.1098/rsif.2017.0213%0A.
Germanotta M, Vasco G, Petrarca M, Rossi S, Carniel S, Bertini E, et al. Robotic and clinical evaluation of upper limb motor performance in patients with Friedreich’s ataxia: an observational study. J Neuroeng Rehabil. 2015;12(1).
Krishnan V, de Freitas PB, Jaric S. Impaired object manipulation in mildly involved individuals with multiple sclerosis. Motor Control 2008;12(1):3–20. Available from: https://doi.org/10.1123/mcj.12.1.3
Rees EM, Farmer R, Cole JH, Haider S, Durr A, Landwehrmeyer B, et al. Cerebellar abnormalities in Huntington’s disease: a role in motor and psychiatric impairment? Mov Disord. 2014;29(13):1648–54.
Koeppen AH, Becker AB, Qian J, Feustel PJ. Friedreich ataxia: hypoplasia of spinal cord and dorsal root ganglia. J Neuropathol Exp Neurol. 2017 12;76(2):nlw111. Available from: https://doi.org/10.1093/jnen/nlw111
Dogan I, Romanzetti S, Didszun C, Mirzazade S, Timmann D, Saft C, et al. Structural characteristics of the central nervous system in Friedreich ataxia: an in vivo spinal cord and brain MRI study. J Neurol Neurosurg Psychiatry. 2018 26;jnnp-2018-318422. Available from: https://doi.org/10.1136/jnnp-2018-318422
Gomes CM, Santos R. Neurodegeneration in Friedreich’s ataxia: from defective frataxin to oxidative stress. Oxid Med Cell Longev. 2013;2013:1–10.
Dumas EM, van den Bogaard SJA, Ruber ME, Reilmann R, Stout JC, Craufurd D, Hicks SL, Kennard C, Tabrizi SJ, van Buchem MA, van der Grond J, Roos RAC Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington’s disease. Hum Brain Mapp 2012 Jan;33(1):203–212. Available from: https://doi.org/10.1002/hbm.21205
Scahill RI, Hobbs NZ, Say MJ, Bechtel N, Henley SMD, Hyare H, et al. Clinical impairment in premanifest and early Huntington’s disease is associated with regionally specific atrophy. Hum Brain Mapp. 2013;34(3):519–29.
Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, Borowsky B, Landwehrmeyer B, Frost C, Johnson H, Craufurd D, Reilmann R, Stout JC, Langbehn DR Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol 2013;12(7):637–649. Available from: https://doi.org/10.1016/S1474-4422(13)70088-7
Reilmann R, Rouzade-Dominguez M-L, Saft C, Süssmuth SD, Priller J, Rosser A, Rickards H, Schöls L, Pezous N, Gasparini F, Johns D, Landwehrmeyer GB, Gomez-Mancilla B A randomized, placebo-controlled trial of AFQ056 for the treatment of chorea in Huntington’s disease. Mov Disord. 2015 Mar;30(3):427–431. Available from: https://doi.org/10.1002/mds.26174
Reilmann R, McGarry A, Landwehrmeyer G, Kieburtz K, Grachev I, Eyal E, et al. Efficacy, safety, and tolerability of pridopidine in Huntington disease (HD): results from the phase II dose-ranging study. Pride-HD Mov Disord. 2017;32(S2):S189.
Funding
KR is funded by the German Federal Ministry of Education and Research (BMBF 01GQ1402). MS received support from the Else Kröner Fresenius Stiftung. ID was supported by the START-Program (08/16) of the Faculty of Medicine at the RWTH Aachen University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
R Schubert is employee of the George-Huntington-Institute and is involved in analysis and development of Q-Motor measures. He received funding from the EU-FP7 consortium REPAIR-HD to develop Q-Motor-based quantitative cognitive assessments.
T Klockgether receives/has received research support from the Deutsche Forschungsgemeinschaft (DFG), the Bundesministerium für Bildung und Forschung (BMBF), the Bundesministerium für Gesundheit (BMG), the Robert Bosch Foundation, the European Union (EU) and the National Institutes of Health (NIH). He has received consulting fees from Biohaven and UBC. He has received a speaker honorarium from Novartis.
R Reilmann is founding director and owner of the George-Huntington-Institute, a private research institute focused on clinical and preclinical research in Huntington disease, and QuantiMedis, a clinical research organisation providing Q-Motor (quantitative motor) services in clinical trials and research. He holds appointments at the Dept. of Radiology of the University of Muenster and at the Department of Neurodegenerative Diseases and Hertie-Institute for Clinical Brain Research, University of Tuebingen. Dr. Reilmann serves as elected member of the Steering Committees of the European Huntington Disease Network (EHDN) and the Huntington Study Group (HSG), co-chair of the Task Force on Huntington’s disease and member of the Task Force on Technology of the International Parkinson and Movement Disorder Society (IPMDS). He has provided consulting services, advisory board functions, clinical trial services, quantitative motor analyses and/or lectures for Actelion Pharmaceuticals, Amarin Neuroscience, AOP Orphan Pharmaceuticals, Cure Huntington Disease Initiative Foundation (CHDI), Desitin, Hoffmann-La Roche, IONIS Pharmaceuticals, Ipsen, Lundbeck, Link Medicine, MEDA Pharma, Medivation, Mitoconix, Neurosearch, Novartis AG, Omeros, Pfizer, Prana Biotechnology, Raptor Pharmaceuticals, Siena Biotech, Temmler Pharma, Teva Pharmaceuticals, uniQure, Vaccinex, Wave Life Sciences and Wyeth Pharmaceuticals. He has received grant support from the Bundesministerium für Bildung und Forschung (BMBF), the Cure Huntington Disease Initiative Foundation (CHDI), the Deutsche Forschungsgemeinschaft (DFG), the Deutsches Zentrum für Neurodegeneration und Entzündung (DZNE), the European Union 7th Framework Program (EU-FP7), the European Huntington Disease Network (EHDN), the High-Q-Foundation and the National Science Foundation (NSF).
All other authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ralf Reilmann and Kathrin Reetz shared last authorships
Electronic Supplementary Material
ESM 1
(DOCX 407 kb)
Rights and permissions
About this article
Cite this article
Hohenfeld, C., Dogan, I., Schubert, R. et al. Application of Quantitative Motor Assessments in Friedreich Ataxia and Evaluation of Their Relation to Clinical Measures. Cerebellum 18, 896–909 (2019). https://doi.org/10.1007/s12311-019-01073-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-019-01073-x