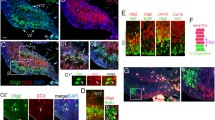
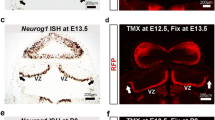
Abstract
The collier/Olf1/EBF family genes encode helix-loop-helix transcription factors (TFs) highly conserved in evolution, initially characterized for their roles in the immune system and in various aspects of neural development. The Early B cell Factor 2 (Ebf2) gene plays an important role in the establishment of cerebellar cortical topography and in Purkinje cell (PC) subtype specification. In the adult cerebellum, Ebf2 is expressed in zebrin II (ZII)-negative PCs, where it suppresses the ZII+ molecular phenotype. However, it is not clear whether Ebf2 is restricted to a PC subset from the onset of its expression or is initially distributed in all PCs and silenced only later in the prospective ZII+ subtype. Moreover, the dynamic distribution and role of Ebf2 in the differentiation of other cerebellar cells remain unclarified. In this paper, by genetic fate mapping, we determine that Ebf2 mRNA is initially found in all PC progenitors, suggesting that unidentified upstream factors silence its expression before completion of embryogenesis. Moreover we show Ebf2 activation in an early born subset of granule cell (GC) precursors homing in the anterior lobe. Conversely, Ebf2 transcription is repressed in other cerebellar cortex interneurons. Last, we show that, although Ebf2 only labels the medial cerebellar nuclei (CN) in the adult cerebellum, the gene is expressed prenatally in projection neurons of all CN. Importantly, in Ebf2 nulls, fastigial nuclei are severely hypocellular, mirroring the defective development of anterior lobe PCs. Our findings further clarify the roles of this terminal selector gene in cerebellar development.






Similar content being viewed by others
References
Armstrong CL, Hawkes R. Pattern formation in the cerebellum: Morgan and Claypool; 2013.
Hawkes R, Gravel C. The modular cerebellum. Prog Neurobiol. 1991;36(4):309–27.
Hawkes R. An anatomical model of cerebellar modules. Prog Brain Res. 1997;114:39–52.
Eisenman LM. Antero-posterior boundaries and compartments in the cerebellum: evidence from selected neurological mutants. Prog Brain Res. 2000;124:23–30.
Sillitoe R, Morphology JA. Molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol. 2007;23:549–77.
Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10(9):670–81.
Apps R, Hawkes R, Aoki S, Bengtsson F, Brown AM, Chen G, et al. Cerebellar modules and their role as operational cerebellar processing units. Cerebellum. 2018.
Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291(4):538–52.
Ahn AH, Dziennis S, Hawkes R, Herrup K. The cloning of zebrin II reveals its identity with aldolase C. Development. 1994;120(8):2081–90.
Chung S-H, Marzban H, Croci L, Consalez G, Hawkes R. Purkinje cell subtype specification in the cerebellar cortex: Ebf2 acts to repress the Zebrin II-positive Purkinje cell phenotype. Neuroscience. 2008;153:721–32.
Croci L, Chung SH, Masserdotti G, Gianola S, Bizzoca A, Gennarini G, et al. A key role for the HLH transcription factor EBF2COE2,O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development. 2006;133(14):2719–29.
Leto K, Arancillo M, Becker EB, Buffo A, Chiang C, Ding B, et al. Consensus paper: cerebellar development. Cerebellum. 2015.
Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, et al. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47(2):201–13.
Pascual M, Abasolo I, Mingorance-Le Meur A, Martinez A, Del Rio JA, Wright CV, et al. Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc Natl Acad Sci U S A. 2007;104(12):5193–8.
Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, et al. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390(6656):169–72.
Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48(1):17–24.
Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48(1):31–43.
Englund C, Kowalczyk T, Daza RA, Dagan A, Lau C, Rose MF, et al. Unipolar brush cells of the cerebellum are produced in the rhombic lip and migrate through developing white matter. J Neurosci. 2006;26(36):9184–95.
Dastjerdi FV, Consalez GG, Hawkes R. Pattern formation during development of the embryonic cerebellum. Front Neuroanat. 2012;6:10.
Mugnaini E, Floris A. The unipolar brush cell: a neglected neuron of the mammalian cerebellar cortex. J Comp Neurol. 1994;339(2):174–80.
Voogd J, Ruigrok TJ. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol. 2004;33(1):5–21.
Lin JC, Cepko CL. Granule cell raphes and parasagittal domains of Purkinje cells: complementary patterns in the developing chick cerebellum. J Neurosci. 1998;18(22):9342–53.
Karam SD, Burrows RC, Logan C, Koblar S, Pasquale EB, Bothwell M. Eph receptors and ephrins in the developing chick cerebellum: relationship to sagittal patterning and granule cell migration. J Neurosci. 2000;20(17):6488–500.
Sillitoe RV, Chung SH, Fritschy JM, Hoy M, Hawkes R. Golgi cell dendrites are restricted by Purkinje cell stripe boundaries in the adult mouse cerebellar cortex. J Neurosci. 2008;28(11):2820–6.
Chung SH, Sillitoe RV, Croci L, Badaloni A, Consalez G, Hawkes R. Purkinje cell phenotype restricts the distribution of unipolar brush cells. Neuroscience. 2009;164(4):1496–508.
Consalez GG, Hawkes R. The compartmental restriction of cerebellar interneurons. Front Neural Circuits. 2012;6:123.
Scott TG. A unique pattern of localization within the cerebellum. Nature. 1963;200:793.
Liao D. Emerging roles of the EBF family of transcription factors in tumor suppression. Mol Cancer Res. 2009;7(12):1893–901.
Herman RK. Mosaic analysis of two genes that affect nervous system structure in Caenorhabditis elegans. Genetics. 1987;116(3):377–88.
Crozatier M, Valle D, Dubois L, Ibnsouda S, Vincent A. collier, a novel regulator of Drosophila head development is expressed in a single mitotic domain. Curr Biol. 1996;6:707–18.
Dubois L, Bally-Cuif L, Crozatier M, Moreau J, Paquereau L, Vincent L. XCoe2, a transcription factor of the Col/Olf-1/EBF family involved in the specification of primary neurons in Xenopus. Curr Biol. 1998;8:199–209.
Pozzoli O, Bosetti A, Croci L, Consalez GG, Vetter ML. Xebf3 is a regulator of neuronal differentiation during primary neurogenesis in Xenopus. Dev Biol. 2001;233(2):495–512.
Hagman J, Belanger C, Travis A, Turck C, Grosschedl R. Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 1993;7:760–73.
Garcia-Dominguez M, Poquet C, Garel S, Charnay P. Ebf gene function is required for coupling neuronal differentiation and cell cycle exit. Development. 2003;130(24):6013–25.
Kratsios P, Kerk SY, Catela C, Liang J, Vidal B, Bayer EA, et al. An intersectional gene regulatory strategy defines subclass diversity of C. elegans motor neurons. eLife. 2017;6.
Catela C, Correa E, Wen K, Aburas J, Croci L, Consalez G, et al. An ancient role for collier/Olf/Ebf (COE)-type transcription factors in axial motor neuron development. Neural Dev. 2019;14(2):2.
Garel S, Marin F, Grosscheld R, Charnay P. Ebf1 controls early cell differentiation in the embryonic striatum. Development. 1999;126(23):5285–94.
Green YS, Vetter ML. EBF factors drive expression of multiple classes of target genes governing neuronal development. Neural Dev. 2011;6:19.
Chiara F, Badaloni A, Croci L, Yeh ML, Cariboni A, Hoerder-Suabedissen A, et al. Early B-cell factors 2 and 3 (EBF2/3) regulate early migration of Cajal-Retzius cells from the cortical hem. Dev Biol. 2012;365(1):277–89.
Corradi A, Croci L, Broccoli V, Zecchini S, Previtali S, Wurst W, et al. Hypogonadotropic hypogonadism and peripheral neuropathy in Ebf2-null mice. Development. 2003;130(2):401–10.
Prasad BC, Ye B, Zackhary R, Schrader K, Seydoux G, Reed RR. Unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O/E family of transcription factors. Development. 1998;125(8):1561–8.
Malgaretti N, Pozzoli O, Bosetti A, Corradi A, Ciarmatori S, Panigada M, et al. Mmot1, a new helix-loop-helix transcription factor gene displaying a sharp expression boundary in the embryonic mouse brain. J Biol Chem. 1997;272(28):17632–9.
Garel S, Marin F, Mattei MG, Vesque C, Vincent A, Charnay P. Family of Ebf/Olf-1-related genes potentially involved in neuronal differentiation and regional specification in the central nervous system. Dev Dyn. 1997;210(3):191–205.
Wang SS, Tsai RYL, Reed RR. The characterization of the Olf-1/EBF-like HLH transcription factor family: implications in olfactory gene regulation and neuronal development. J Neurosci. 1997;17:4149–58.
Moruzzo D, Nobbio L, Sterlini B, Consalez GG, Benfenati F, Schenone A, et al. The transcription factors EBF1 and EBF2 are positive regulators of myelination in Schwann cells. Mol Neurobiol. 2016;54(10):8117–27.
Giacomini C, La Padula V, Schenone A, Leandri M, Contestabile A, Moruzzo D, et al. Both Schwann cell and axonal defects cause motor peripheral neuropathy in Ebf2−/− mice. Neurobiol Dis. 2011;42(1):73–84.
Croci L, Barili V, Chia D, Massimino L, van Vugt R, Masserdotti G, et al. Local insulin-like growth factor I expression is essential for Purkinje neuron survival at birth. Cell Death Differ. 2011;18(1):48–59.
Hoxha E, Tonini R, Montarolo F, Croci L, Consalez GG, Tempia F. Motor dysfunction and cerebellar Purkinje cell firing impairment in Ebf2 null mice. Mol Cell Neurosci. 2013;52:51–61.
Bizzoca A, Picocci S, Corsi P, Arbia S, Croci L, Consalez GG, et al. The gene encoding the mouse contactin-1 axonal glycoprotein is regulated by the collier/Olf1/EBF family early B-cell factor 2 transcription factor. Dev Neurobiol. 2015;75(12):1420–40.
Chung SH, Marzban H, Hawkes R. Compartmentation of the cerebellar nuclei of the mouse. Neuroscience. 2009;161(1):123–38.
Copeland NG, Jenkins NA, Court DL. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet. 2001;2(10):769–79.
Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33(4):e36.
Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E, et al. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32(1):19–26.
Qian H, Badaloni A, Chiara F, Stjernberg J, Polisetti N, Nihlberg K, et al. Molecular characterization of prospectively isolated multipotent mesenchymal progenitors provides new insight into the cellular identity of mesenchymal stem cells in mouse bone marrow. Mol Cell Biol. 2013;33(4):661–77.
Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1.
Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4.
Buffo A, Rossi F. Origin, lineage and function of cerebellar glia. Prog Neurobiol. 2013;109:42–63.
Kratsios P, Stolfi A, Levine M, Hobert O. Coordinated regulation of cholinergic motor neuron traits through a conserved terminal selector gene. Nat Neurosci. 2011;15(2):205–14.
Baumgardt M, Karlsson D, Terriente J, Diaz-Benjumea FJ, Thor S. Neuronal subtype specification within a lineage by opposing temporal feed-forward loops. Cell. 2009;139(5):969–82.
Masserdotti G, Badaloni A, Green YS, Croci L, Barili V, Bergamini G, et al. ZFP423 coordinates Notch and bone morphogenetic protein signaling, selectively up-regulating Hes5 gene expression. J Biol Chem. 2010;285(40):30814–24.
Tsai RYL, Reed RR. Identification of DNA recognition sequences and protein interaction domains of the multiple zinc finger protein Roaz. Mol Cell Biol. 1998;18:6447–56.
Tsai RY, Reed RR. Cloning and functional characterization of Roaz, a zinc finger protein that interacts with O/E-1 to regulate gene expression: implications for olfactory neuronal development. J Neurosci. 1997;17(11):4159–69.
Casoni F, Croci L, Cremona O, Hawkes R, Consalez G. Early Purkinje cell development and the origin of cerebellar patterning. In: Marzban H, editor. Development of the cerebellum, from molecular aspects to diseases. Cham: Springer Nature; 2017. p. 67–86.
Frantz GD, Weimann JM, Levin ME, McConnell SK. Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum. J Neurosci. 1994;14(10):5725–40.
Hawkes R, Beierbach E, Tan SS. Granule cell dispersion is restricted across transverse boundaries in mouse chimeras. Eur J Neurosci. 1999;11(11):3800–8.
Miyata T, Maeda T, Lee JE. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 1999;13(13):1647–52.
Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27(36):9780–9.
Acknowledgments
Oocyte injections were performed at the Center for Conditional Mutagenesis (CFCM), San Raffaele Scientific Institute. Image analysis was carried out at ALEMBIC, an advanced microscopy laboratory established by the San Raffaele Scientific Institute and University.
Funding
G.G.C.’s research was funded by the Italian Telethon Foundation, grant GGP13146. O.C. was the recipient of a grant from the Italian Ministry of Health (Ministero della Salute Ricerca Finalizzata 2011-PE-2011-02347716). R.H. was supported by an award from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experimental plan was designed in agreement with the stipulations of the San Raffaele Institutional Animal Care and Use Committee (permit number 336).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Badaloni, A., Casoni, F., Croci, L. et al. Dynamic Expression and New Functions of Early B Cell Factor 2 in Cerebellar Development. Cerebellum 18, 999–1010 (2019). https://doi.org/10.1007/s12311-019-01051-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-019-01051-3