Abstract
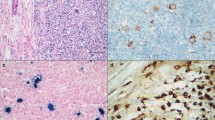
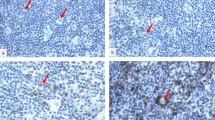
The effect of IgG4, which constitutes the least of the IgG subclasses, on the pathogenesis and prognosis of lymphoma or solid tumors is one of the research topics of interest in recent years. The role of IgG4, which has been reported to suppress antitumor immunity, in classic Hodgkin’s lymphoma (cHL), which is recognized by its pathognomonic microenvironment, is not yet clearly known. The aim of this study was to determine IgG4-positive plasma cell density in the cHL microenvironment and to compare it with histopathological and clinical parameters. In addition, the role of the increase in IgG4-positive cells in the development of relapse after treatment was also investigated. A retrospective cross-sectional study. Ninety-four patients with the initial diagnosis of cHL who had no comorbidity or no treatment history and forty-one reactive lymph nodes with follicular hyperplasia findings were included in the study. Three hot-spot areas were identified with reference to the IgG4 sections. Mean IgG4-positive plasmacyte counts and IgG4/IgG ratios were determined and compared with histopathological characteristics. The mean IgG4 + plasma cell count was 33.57 in cHL cases and 47.04 in the control group (p = 0.233). IgG4/IgG ratio was significantly higher in cHL compared with the control group (0.27 vs. 0.21, p = 0.021). The IgG4/IgG ratio was found to be higher in younger patients with classic Hodgkin lymphoma, with a low correlation (p = 0.028, r = − 0.226). There was no relationship with gender, lymph node location, histological subtype, EBV positivity and bone marrow infiltration. It was observed that IgG4/IgG ratio was higher in early-stage patients (p = 0.022). No significant IgG4 + cell increase was detected in the initial diagnosis and relapse slides of six patients who developed relapse after standard treatment, resulting in a cure. Novel therapeutic modalities targeting microenvironmental components have been reported to show dramatic effects, particularly in relapsed or refractory patients. Detailed characterization of the cHL inflammatory milieu will be useful for the identification of alternative targets. IgG4 subclass antibodies, which have been described to have anti-inflammatory effects, may have prognostic significance in a proportion of cHL patients.



Similar content being viewed by others
Data Availability
This study was funded by the Scientific Research Projects (BAP) Coordination Unit of Bezmialem Vakif University.
References
Aldinucci D, Borghese C, Casagrande N (2019) Formation of the immunosuppressive microenvironment of classic Hodgkin lymphoma and therapeutic approaches to counter it. Int J Mol Sci 20:2416. https://doi.org/10.3390/ijms20102416
Weniger MA, Küppers R (2021) Molecular biology of Hodgkin lymphoma. Leukemia 35:968–981. https://doi.org/10.1038/s41375-021-01204-6
Wang HW, Balakrishna JP, Pittaluga S, Jaffe ES (2019) Diagnosis of Hodgkin lymphoma in the modern era. Br J Haematol 184:45–59. https://doi.org/10.1111/bjh.15614
Cader FZ, Schackmann RC, Hu X, Wienand K, Redd R, Chapuy B et al (2018) Mass cytometry of Hodgkin lymphoma reveals a CD4+ regulatory T-cell–rich and exhausted T-effector microenvironment. Blood 132:825–836. https://doi.org/10.1182/blood-2018-04-843714
Greaves P, Clear A, Owen A, Iqbal S, Lee A, Matthews J et al (2013) Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood 122:2856–2863. https://doi.org/10.1182/blood-2013-06-508044
Patel SS, Weirather JL, Lipschitz M, Lako A, Chen P-H, Griffin GK et al (2019) The microenvironmental niche in classic Hodgkin lymphoma is enriched for CTLA-4–positive T cells that are PD-1–negative. Blood 134:2059–2069. https://doi.org/10.1182/blood.2019002206
Gholiha AR, Hollander P, Hedstrom G, Sundstrom C, Molin D, Smedby KE et al (2019) High tumour plasma cell infiltration reflects an important microenvironmental component in classic Hodgkin lymphoma linked to presence of B-symptoms. Br J Haematol 184:192–201. https://doi.org/10.1111/bjh.15703
Ahn SS, Song JJ, Park YB, Lee SW (2017) Malignancies in Korean patients with immunoglobulin G4-related disease. Int J Rheum Dis 20:1028–1035. https://doi.org/10.1111/1756-185X.13093
Kiil K, Bein J, Schuhmacher B, Thurner L, Schneider M, Hansmann M-L et al (2018) A high number of IgG4-positive plasma cells rules out nodular lymphocyte predominant Hodgkin lymphoma. Virchows Arch 473:759–764. https://doi.org/10.1007/s00428-018-2460-8
Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L et al (2013) IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest 123:1457–1474. https://doi.org/10.1172/JCI65579
Yamamoto M, Takahashi H, Tabeya T, Suzuki C, Naishiro Y, Ishigami K et al (2012) Risk of malignancies in IgG4-related disease. Mod Rheumatol 22:414–418. https://doi.org/10.1007/s10165-011-0520-x
Sohn EJ, Ahn HB, Roh MS, Jung WJ, Ryu WY, Kwon YH (2018) Immunoglobulin G4 (IgG4)-positive ocular adnexal mucosa-associated lymphoid tissue lymphoma and idiopathic orbital inflammation. Ophthalmic Plast Reconstr Surg 34:313–319. https://doi.org/10.1097/IOP.0000000000000965
Bledsoe JR, Wallace ZS, Stone JH, Deshpande V, Ferry JA (2018) Lymphomas in IgG4-related disease: clinicopathologic features in a Western population. Virchows Arch 472:839–852. https://doi.org/10.1007/s00428-017-2286-9
Ferry JA (2013) IgG4-related lymphadenopathy and IgG4-related lymphoma: moving targets. Diagn Histopathol 19:128–139. https://doi.org/10.1016/j.mpdhp.2013.01.005
Takahashi N, Ghazale AH, Smyrk TC, Mandrekar JN, Chari ST (2009) Possible association between IgG4-associated systemic disease with or without autoimmune pancreatitis and non-Hodgkin lymphoma. Pancreas 38:523–526. https://doi.org/10.1097/MPA.0b013e31819d73ca
Nowak V, Agaimy A, Kristiansen G, Gütgemann I (2020) Increased IgG4-positive plasma cells in nodular-sclerosing Hodgkin lymphoma: a diagnostic pitfall. Histopathology 76:244–250. https://doi.org/10.1111/his.13965
Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T et al (2012) Consensus statement on the pathology of IgG4-related disease. Mod Pathol 25:1181–92. https://doi.org/10.1038/modpathol.2012.72
Glimelius I, Diepstra A (2017) Novel treatment concepts in Hodgkin lymphoma. J Intern Med 281:247–260. https://doi.org/10.1111/joim.12582
Brice P, de Kerviler E, Friedberg JW (2021) Classical Hodgkin lymphoma. Lancet 398:1518–1527. https://doi.org/10.1016/S0140-6736(20)32207-8
Nishikori A, Nishimura Y, Shibata R, Ohshima K-i, Gion Y, Ikeda T et al (2021) Upregulated expression of activation-induced cytidine deaminase in ocular adnexal marginal zone lymphoma with IgG4-positive cells. Int J Mol Sci. 22:4083. https://doi.org/10.3390/ijms22084083
Cheuk W, Tam FK, Chan AN, Luk IS, Yuen AP, Chan W-K et al (2010) Idiopathic cervical fibrosis—a new member of IgG4-related sclerosing diseases: report of 4 cases, 1 complicated by composite lymphoma. Am J Surg Pathol 34:1678–1685. https://doi.org/10.1097/PAS.0b013e3181f12c85
Vardhana S, Younes A (2016) The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica 101:794. https://doi.org/10.3324/haematol.2015.132761
Bianchini R, Karagiannis SN, Jordakieva G, Jensen-Jarolim E (2020) The role of IgG4 in the fine tuning of tolerance in IgE-mediated allergy and cancer. Int J Mol Sci 21:5017. https://doi.org/10.3390/ijms21145017
Sato Y, Notohara K, Kojima M, Takata K, Masaki Y, Yoshino T (2010) IgG4-related disease: historical overview and pathology of hematological disorders. Pathol Int 60:247–258. https://doi.org/10.1111/j.1440-1827.2010.02524.x
Cheuk W, Yuen HK, Chan AC, Shih L-Y, Kuo T-T, Ma M-W et al (2008) Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol 32:1159–1167. https://doi.org/10.1097/PAS.0b013e31816148ad
Mitsui T, Yokohama A, Koiso H, Ishizaki T, Uchiumi H, Saitoh T et al (2013) Development of IgG4-related disease 10 years after chemotherapy for diffuse large B cell lymphoma and longstanding bronchial asthma. Int J Hematol 98:122–128. https://doi.org/10.1007/s12185-013-1359-z
Matsuo T, Ichimura K, Yoshino T (2011) Local recurrence as immunoglobulin G4 (IgG4)-related disease 10 years after radiotherapy to ocular adnexal extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. J Clin Exp Hematop 51:125–133. https://doi.org/10.3960/jslrt.51.125
Funding
This study was funded by the Scientific Research Projects (BAP) Coordination Unit of our university.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This study has obtained IRB approval from by the ethics committee of our university (E-54022451–050.05.04–96966) and the need for informed consent was waived.
Consent for publication
For this type of study, consent for publication is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guler, B., Tekden, B.C., Cetin, G. et al. The role of IgG4-positive plasma cell population in classic Hodgkin lymphoma. J Hematopathol 16, 191–197 (2023). https://doi.org/10.1007/s12308-023-00559-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-023-00559-2