Abstract
Peripheral T cell lymphomas are clinically and pathologically complex and generally associated with overall poor prognosis and aggressive clinical course. In recent years, there is a greater recognition of abnormal B cell expansion as a component of T cell lymphomas especially those derived from follicular helper T cells. Most of these B cells are EBV positive and show a wide range of morphology which includes large mononuclear cells and Hodgkin-like cells. The number of the abnormal B cells can also vary. It is possible to misdiagnose this entity as a B cell lymphoma, Hodgkin lymphoma, composite lymphoma, or reactive lymphoid proliferation based on the number and morphology of the proliferating B cells. Herein, we report the case of an 82-year-old woman who presented with cervical lymphadenopathy, excision biopsy of which showed diffusely arranged atypical small- to medium-sized cells with irregular nuclei admixed with large number of immunoblast-like large cells. Immunophenotyping showed the small- to medium-sized cells to be CD20 negative, CD3 positive, and CD5 positive and showed downregulation of CD7. These cells were CD25 positive and showed a high MIB 1 labelling index. The large cells were CD20 positive and CD30 positive and showed EBV-encoded small nuclear RNA (EBER) positivity. Serum HTLV-1 estimation was positive. Molecular studies showed TCR gene rearrangement and a polyclonal population of B cells. Based on morphology, immunoprofile, and molecular studies, a diagnosis of adult T cell leukemia/lymphoma complicated by proliferation of large B cells was given. The presence of large B cell proliferation in adult T cell leukemia/lymphoma is an exceptionally rare phenomenon.
Similar content being viewed by others
Introduction
Proliferations of Epstein-Barr virus (EBV)-positive large B cells as a component of T cell lymphomas are being increasingly recognized [1,2,3,4,5]. This phenomenon has been described in association with lymphomas derived from follicular helper T cells like peripheral T cell lymphoma not otherwise specified (PTCL NOS) and angioimmunoblastic T cell lymphoma (AITL) [1, 4]. The follicular helper T cells may facilitate the expansion of abnormal B cells through an immunological blockade [4]. The marked immunosuppression in T cell lymphoma may be the cause of EBV-positive B cell proliferation. The transformed B cells vary in morphology, immunophenotype, and genotype. The diagnosis of T cell lymphoma complicated by a proliferation of large B cells depends on extensive immunophenotypic and molecular evaluation. This large B cell-rich T cell lymphoma should not be mistaken for a large B cell lymphoproliferative disorder [2]. Because of the presence of large B and T cells along with positivity for EBV, there is a chance for misdiagnosis as a reactive process. The awareness of the phenomenon and the use of immunohistochemistry and molecular studies will help to render an accurate diagnosis.
Case report
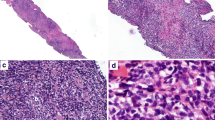
An 82-year-old female patient presented with cervical lymph node enlargement of 3-month duration. Excision biopsy of the node was done in an outside center. We reviewed the slides. Histopathological examination showed diffusely arranged small- to medium-sized atypical cells with irregular nuclei (Fig. 1). There was another population of large atypical lymphoid cells with moderate amount of cytoplasm, round/slightly indented nuclei, and eosinophilic nucleoli. The large cells were immunoblast-like cells and were seen intermingled with the small atypical cells. On immunohistochemical evaluation, the small- to medium-sized atypical cells were CD20 negative, CD10 negative, BCL6 negative, CD3 positive, and CD5 positive and showed downregulation of CD7 (Fig. 2). These cells showed positivity for CD25 and showed a high MIB 1 labelling index. The large cells were positive for CD20, CD30, and EBV-encoded small nuclear RNA (EBER) and were CD15 negative (Fig. 3). On further detailed evaluation, the patient gave a history of small hyperpigmented lesions in the skin. There were multiple enlarged cervical lymph nodes. Peripheral smear showed very few atypical lymphocytes with convoluted nuclei. A diagnosis of adult T cell leukemia/lymphoma was confirmed by positive serum HTLV-1 estimation. In this point, we could not ascertain the nature of B cell proliferation. To differentiate between a composite lymphoma and a B cell proliferation associated with T cell lymphoma, molecular studies were advised which showed TCR gene rearrangement and polyclonal B cell population. With these findings, a diagnosis of adult T cell leukemia/lymphoma with associated proliferation of large B cells was given.
Discussion
Proliferation of Epstein-Barr virus (EBV)-positive B cells is being increasingly recognized in T cell lymphomas [1, 2, 4]. When there is florid proliferation of large B cells, there is a greater chance of these neoplasms being misdiagnosed as large B cell lymphoma and the diagnosis of a T cell lymphoma is made only on recurrence. As the clinical management is entirely different in T cell and B cell lymphomas, the awareness of this phenomenon is very important. On the other end of the spectrum, the histological appearance and immunoprofile showing a mixture of T cells and CD30-positive large B cells, a misdiagnosis of a reactive lymphoid proliferation is a possibility.
The phenomenon of large B cell proliferation in T cell lymphomas is commonly seen in lymphomas derived from T follicular helper cells (TFH cells) [1,2,3,4,5]. TFH cells are a unique subset of T cells found in normal germinal center. These cells provide help to B cells in germinal center reactions and express markers such as BCL6, CD10, CD4, PD-1, SAP, and IL-2. TFH cells produce a chemokine receptor CXCR5 and chemokine CXCL13 which cause induction and proliferation of FDC [4]. It facilitates the adhesion of B cells to high endothelial venule and is involved in the recruitment of B cells to the lymph node. TFH cells play critical role in T cell-dependent B cell response. These cells promote the expansion of B cells in immune response. The continuing TFH cell help in TFH cell lymphomas may aberrantly expand the B cells outside normal physiological control. TFH-derived neoplasms such as angioimmunoblastic T cell lymphoma, follicular variant of PTCL NOS, and primary cutaneous small/medium CD4-positive T cell lymphomas are often associated with atypical B cell proliferation [4]. Polyclonal hypergammaglobulinemia and polyclonal plasmacytosis are often observed in AITL cases. Rarely, the marked B cell or plasma cell expansion can obscure the underlying T cell neoplasm [6]. EBV-positive B cells with immunoblastic features are nearly always found in the background of AITL cases [5]. In their series of AITL with a large B cell proliferation, Lome-Maldonado et al. noted that the presence of large B cells made no prognostic impact and they proposed AITL with large B cell proliferation as a specific subset of AITL [7]. A dramatic increase in the number of large cells has been reported in cases of PTCL NOS and is termed PTCL complicated by a proliferation of large B cells [1, 5, 7].
In most of the cases, the large B cell population is EBV infected [2, 4]. The expansion of EBV-positive B cells may be related to the defective immune surveillance secondary to T cell lymphomas. The relatively immunocompromised status in T cell lymphomas may facilitate EBV reactivation that subsequently lead to transformation of EBV-infected B cells through a polyclonal-oligoclonal-monoclonal program as in post-transplant lymphoproliferative disorder [3, 4]. It is postulated that in EBV-negative cases, the neoplastic T cells function as helper cells to promote the B cell proliferation.
EBV-negative clonal or monocytic B cell proliferations in patients with PTCL range from plasma cell proliferation to overt lymphomas. In their series, Balague et al. describe EBV-negative clonal plasma cell proliferation and lymphomas in 15 PTCL cases [8]. They observed clonal or monocytic plasma cell proliferations in eight cases, clonal or monocytic B cell proliferations in four cases, and B cell lymphoma with plasmacytic or plasmablastic differentiation in three cases.
B cell proliferation in cases of HTLV-1-positive adult T cell leukemia/lymphoma (ATLL) is an extremely rare phenomenon [9, 10]. In ATLL, there is a marked impairment of the immune system and these patients have a 25 fold increased risk of opportunistic infections compared to other types of non-Hodgkin lymphomas. ATLL has been linked to the Treg cells, which are a special type of regulatory T cells that suppresses the immune response and this explains the marked immunosuppression associated with ATLL. This marked defect in immune surveillance may be the reason for the proliferation of EBV-positive large B cells in ATLL cases [10]. Katsuki et al. demonstrated defective cytotoxic T lymphocytes (CTL) in ATLL patients. In their study, CTL obtained from nine EBV-seropositive ATLL patients were unable to induce regression of foci of EBV-transformed cells in vitro. The same assay using CTL from 10 healthy EBV-seropositive patients resulted in regression of the foci [11].
In majority of the cases of T cell lymphomas with proliferation of large B cells, the large cells have morphology similar to immunoblasts or Hodgkin cells. The numbers of large cells also vary and most of the authors require more than 25% of the background cells to be the large cells to call a significant large B cell proliferation [1, 7]. The EBV-infected Hodgkin-like cells will express CD30 mimicking classical Hodgkin lymphoma [4]. But the B cell program is most often preserved and will show strong expression of CD20 and PAX5, which facilitate to differentiate from classical Hodgkin lymphoma.
The B cells may show polyclonal, oligoclonal, or monoclonal immunoglobulin heavy chain rearrangement [1, 5]. If there are no overt features of B cell lymphoma like sheeting of the large B cells and architectural destruction, it is recommended to label the cases as PTCL complicated by a proliferation of large B cells and note the immunoglobulin gene rearrangement findings [1]. Cases showing architectural destruction and sheeting of B cells along with monoclonality should be classified as composite lymphoma. Rare cases of concurrent angioimmunoblastic T cell lymphoma and large B cell lymphoma, and the occurrence of B cell lymphoma after AILT has been reported [1, 12].
The phenomenon of marked proliferation of large B cells in T cell lymphomas can be diagnostically challenging. It can be mistaken as a reactive process when there is proliferation of EBV-positive large B cells with immunoblastic morphology, as Hodgkin lymphoma when the B cells show a Hodgkin cell-like morphology along with positivity for CD30 and EBER, or as a T cell-rich large B cell lymphoma when there is a clustering of large B cells. Extensive morphologic, immunophenotypic, and molecular evaluation along with the awareness of the entity will help to render an accurate diagnosis.
References
Reichard KK, Schwartz EJ, Higgins JP, Narasimhan B, Warnke RA, Natkunam Y (2006) CD10 expression in peripheral T-cell lymphomas complicated by a proliferation of large B-cells. Mod Pathol 19:337–343
Higgins JP, van de Rijn M, Jones CD, Zehnder JL, Warnke RA (2000) Peripheral T-cell lymphoma complicated by a proliferation of large B cells. Am J Clin Pathol 114:236–247
Willenbrock K, Brauninger A, Hansmann ML (2007) Frequent occurrence of B-cell lymphomas in angioimmunoblastic T-cell lymphoma and proliferation of Epstein-Barr virus-infected cells in early cases. Br J Haematol 138:733–739
Nicolae A, Pittaluga S, Venkataraman G, Vijnovich- Baron A, Xi L, Raffeld M et al (2013) Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: both EBV-positive and EBV-negative variants exist. Am J Surg Pathol 37:816–826
Jaffe ES, Nicolae A, Pittaluga S (2013) Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol 26:S71–S87
Huppmann AR, Roullet MR, Raffeld M, Jaffe ES (2013) Angioimmunoblastic T-cell lymphoma partially obscured by an Epstein-Barr virus-negative clonal plasma cell proliferation. J Clin Oncol 31:e28–e30
Lome-Maldonado C, Canioni D, Hermine O, Delaesse E, Damotte D, Raffoux E et al (2002) Angioimmunoblastic T cell lymphoma (AILD-TL) rich in large B cells and associated with Epstein-Barr virus infection. A different subtype of AILD-TL? Leukemia 16:2134–2141
Balague O, Martinez A, Colomo L, Rosello E, Garcia A et al (2007) Epstein-Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: a phenomenon with distinctive clinicopathologic features. Am J Surg Pathol 31:1310–1322
Venkataraman G, Berkowitz J, Morris JC, Janik JE, Raffeld MA, Pittaluga S (2011) Adult T-cell leukemia/lymphoma with Epstein-Barr virus-positive Hodgkin-like cells. Hum Pathol 42:1042–1046
Ohshima K, Karube K, Hamasaki M, Suefuji H, Tutiya T, Yamaguchi T et al (2003) Imbalances of chemokines, chemokine receptors and cytokines in Hodgkin lymphoma: classical Hodgkin lymphoma vs. Hodgkin-like ATLL. Int J Cancer 106:706–712
Katsuki T, Yamaguchi K, Matsuoka Y, Hinuma Y (1986) Impairment of T-cell control of Epstein-Barr virus infected B-cells in patients with adult T-cell leukemia. AIDS Res 2:S125–S130
Xu Y, McKenna RW, Hoang MP, Collins RH, Kroft SH (2002) Composite angioimmunoblastic T cell lymphoma and diffuse large B- cell lymphoma. Am J Clin Pathol 118:848–854
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sukumaran, R., Nair, R.A. Adult T cell leukemia/lymphoma complicated by proliferation of large B cells: a diagnostic dilemma. J Hematopathol 11, 83–86 (2018). https://doi.org/10.1007/s12308-018-0326-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-018-0326-2