Abstract
Introduction
Detecting low viral load has been a challenge in this pandemic, which has led to its escalated transmission. Complement activation has been implicated in pathogenesis of Covid-19 infection. Thus, evaluation of complement activation in suspected Covid-19 infection may help to detect infection and limit false negative cases thus limiting transmission of infection. We speculate that measuring C4b, produced from an activated complement system due to the presence of Covid-19 may help in its detection, even when the viral titers are low.
Methods
Plasma C4b levels of symptomatic RT-PCR positive patients (cases, n = 40); symptomatic RT-PCR negative patients (n = 35) and asymptomatic RT-PCR negative controls (n = 40) were evaluated. Plasma C5b-9, IL-6, D-dimer and C1-Inhibitor (C1-INH) were also measured in cases and controls. ELISA kits were used for all measurements. Statistical analyses were carried out using Stata, version 12 (Stata Corp., Texas, USA).
Results
C4b levels were found to be significantly increased in RT-PCR positive patients as compared to asymptomatic RT-PCR negative controls. RT-PCR negative but symptomatic patients still showed increased C4b levels. The significantly higher levels of C4b in cases with a cut-off value of ≥ 116 ng/ml with optimum sensitivity and specificity of 80% and 52% respectively is indicative of its possible use as an adjunct marker. Increased levels of D-dimer, IL6, along with decreased levels of C1-INH were found in cases compared to controls. Whereas, C5b-9 levels were not significantly raised in cases.
Conclusions
The results of our study suggests that plasma C4b may help to detect infection in false negative cases of RT-PCR that escape detection owing to low viral load. However, to confirm it a large-scale study is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) referred to as Covid-19 has emerged as a public health emergency with high morbidity and mortality [1,2,3]. This virus has been reported to be highly infective and limiting its transmission has been a challenge in this pandemic. Timely diagnosis of corona-infected cases is the most essential part of prevention of disease. The most established diagnostic test to Covid-19 infection is the RT-PCR test, which detects the viral nucleic acid while other tests detect either viral protein(s) or antibodies against viral protein(s). There have been evidences where the RT-PCR test has been reported to have low sensitivity and cases with low viremia load escape detection by this test [4, 5].
In severe cases of Covid-19 infection, the pathologies are mediated by immune responses with the complement system playing the key role [6]. Many studies have unfolded the possible role of over-activated complement pathway(s) in acute and chronic inflammatory response, tissue injury and coagulopathy observed in Covid-19 patients [6]. Evidence of complement activation in lung tissue, skin, and sera has been reported. Also, the treatment with complement inhibitors helped in recovery without any adverse reactions [7]. Hence, complement activation may become instrumental in detecting the infection by measuring some complement fragment produced as a result of its activation due to presence of virus. Generation of complement fragment C4b from activated C4 has been reported in viral infections [8, 9]. Also, activation of Lectin Pathway by recombinant SARS-CoV-2 proteins has been reported to bind C4b [10].
It is important to note that C4b levels have never been looked for complement activation in Covid-19 patients till date. We speculate that measuring C4b, produced from an activated complement system due to the presence of Covid-19 may help in its detection, even when the viral titers are low.
Further, though the vaccines against Covid-19 have provided protection from the disease and the rate of viral transmission has dropped significantly [11], detection of Covid-19 infection in the vaccinated population with mild symptoms due to low viral titers may pose a challenge. Studies have reported that increased viral load is associated with Covid-19 symptoms as well as with duration since infection [12,13,14,15]. The immunized host with a primed immune system against the virus doesn’t allow the virus to replicate that fast when infected, and maintain low viral titers with only mild symptoms [16] and may escape detection. Hence, diagnosing such cases is important to further limit the transmission. In view of this, we evaluated C4b levels in cases, controls and vaccinated individuals who had flu-like symptoms but tested negative with RT-PCR. Other markers of Covid-19 infection like C5b-9, IL-6, D-dimer, and C1-INH were also investigated in this study.
Materials and methods
Study participants
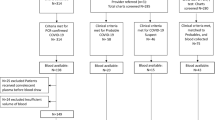
The study consisted of 40 cases and 40 control samples. Cases were mild symptomatic, stable patients, tested positive with RT-PCR (PCR cycle threshold value for positivity of infection was less than 35 in all samples) and admitted to hospital whereas controls were RT-PCR negative and without any flu-like symptoms. Later, another 35 participants were recruited who tested negative with RT-PCR but had flu-like symptoms as observed in Covid-19 patients. This group of participants were mostly vaccinated (n = 25) with Covaxin and few had only the first dose of the same vaccine (n = 10) (Table 1). Most of them completed vaccination in the months of February and March 2021.
The study protocol was approved by the Institutional ethical committee of All India Institute of Medical Sciences, Patna, Bihar, India and informed consent were obtained from all the participants.
Table 1 shows the detailed information of participants.
Sample collection
As per Institute’s guidelines, the institute’s employees who were either symptomatic or had exposure to high risk confirmed positive cases of Coronavirus were tested by RT-PCR at the Institute’s laboratory. A database of the same was available for the research team, which was utilized for collection of samples. The major part of the study was conducted from October to December 2020. A part of study (with 35 samples) was done in the month of July 2021.
All test samples were collected in ethylenediamine tetra-acetic acid (EDTA) anticoagulant vials and were stored at 4 °C. These were centrifuged at 3000 rpm for 10 min within 1 h after venipuncture and plasma was stored at -20 °C for further analysis. Levels of C4b, C5b-9, C1-INH, D-dimer and IL6 were evaluated in samples collected in the cases and control group. However, C4b levels were also measured in symptomatic patients, tested negative with RT-PCR test.
Measurement of C4b, C5b-9 and C1-INH
Levels of C4b, C5b-9 and C1-INH were evaluated by ELISA kits (Bioassay Technology, Shanghai Korain Biotech Co) according to manufacturer’s guidelines with some minor modifications. In brief, 40 µl of plasma sample, 10 µl of biotinylated human antibody (against the specific protein), and 50 µl of streptavidin Horseradish Peroxidase (HRP) were added to the pre-coated plate with specific antibody. This was incubated at 37 °C for 60 min and washed with a 1X washing buffer using an automated ELISA washer (SW40, Bio-Rad California, USA). Further, 50 µl of substrate solution ‘A’ and ‘B’ were added and incubated at 37 °C for 10 min. Finally, 50 µl of H2O2 solution was added and optical density (OD) at 450 nm was taken within 10 min of addition of H2O2, using microplate reader (PR4100 Bio-Rad USA). The calculation was performed with computer-based curve-fitting software (Magellan, PR4100 Bio-Red, USA).
Evaluation of D-dimer
The levels of D-dimer were evaluated by Immuno-turbidometry assay by STA-Liatest D-Di plus kit. This assay is based on measurement of change in turbidity of a microparticle suspension by photometry.
A suspension of latex microparticles, coated with monoclonal antibodies specific for D-dimer, was mixed with the plasma. The turbidity developed due to an antigen-antibody reaction leading to an agglutination of the latex microparticles was measured photometrically as an increase in absorbance.
Evaluation of IL-6
The levels of IL-6 were evaluated using the ADVIA Centaur IL-6 immunoassay kit using chemiluminescent technology. The Solid Phase consisted of anti-IL-6 mouse monoclonal antibody coated paramagnetic microparticles. The plasma sample was incubated with acridinium ester-labelled monoclonal mouse anti-IL-6 antibody as the Lite Reagent and Solid Phase reagent to allow formed immune complexes to be captured by the particles. After incubation, the particles were washed before addition of the ADVIA Centaur Acid Reagent and ADVIA Centaur Base Reagent to initiate the chemiluminescent reaction. A direct relationship exists between the amount of IL-6 present in the sample and the amount of Relative Light Units (RLUs) detected by the system.
Assay Procedure
The system automatically performs the following steps. (i) Dispenses 50 µL of sample into a cuvette. (ii) Dispenses 140 µL of Solid Phase and 100 µL of Lite Reagent, and then incubates for 7.5 min at 37 °C on the ADVIA Centaur XP/XPT systems and 9.7 min at 37 °C on the ADVIA Centaur CP system. (iii) Separates the Solid Phase from the mixture, aspirates the unbound reagent, and washes the cuvettes with ADVIA Centaur Wash 1. (iv) Dispenses 300 µL each of ADVIA Centaur Acid Reagent and ADVIA Centaur Base Reagent to initiate the chemiluminescent reaction. (v) Reports results.
Statistical Analysis
All statistical analyses were carried out using Stata, version 12 (Stata Corp., Texas, USA). Continuous variables were presented as mean with 95% confidence interval (CI). Test of significance based on parametric distributions was applied after checking for the normality conditions of continuous variables. Student’s t-test for difference of mean between two groups was used for comparing mean values of continuous variables. Pearson’s correlation coefficient was computed and t-test for correlation coefficient was applied to test the significance of all computed correlations. Receiver operating characteristics (ROC) curve was used to determine the cut-off values with optimum sensitivity and specificity. Linear regression model was used to model the dependent variable C4b using independent variables like markers of Covid-19, disease status.
Results
To assess complement activation in Covid-19 infection, we measured plasma C4b levels in RT-PCR positive Covid-19 cases, RT-PCR negative asymptomatic controls and RT-PCR negative symptomatic vaccinated cases. The C4b levels were found to be significantly raised in both symptomatic RT-PCR positive and symptomatic RT-PCR negative cases as compared to asymptomatic RT-PCR negative controls (Fig. 1 A/Table 1). The C4b value of 312.49 ng/ml of symptomatic RT-PCR positive and 308.38 ng/ml of symptomatic of RT-PCR negative cases were not significantly different. However, they were statistically significantly different from asymptomatic RT-PCR negative controls (C4b of 72.87 ng/ml) (Fig. 1A). The cutoff value of C4b ≥ 116 ng/ml with optimum sensitivity and specificity 80% and 52% respectively shows its predictive potential in mild cases (Fig. 1A &1B).
Evaluation of plasma C4b levels. (A) Comparison of mean levels of C4b (ng/ml) among asymptomatic RT-PCR negative subjects (0), symptomatic RT-PCR positive (1), and symptomatic RT-PCR negative (2). The values are shown in the insert table with p-values showing the statistical significance among three groups. The mean C4b levels of symptomatic subjects irrespective of RT-PCR positivity were not significantly different (p-value = 1.00), whereas these levels were significantly different in comparison to RT-PCR negative asymptomatic controls. (B) Receiver Operating Characteristic (ROC) curve for C4b with area under curve (AUC): ROC curve was analysed for C4b values in cases and controls. The cases were RT-PCR positive with symptoms while controls included RT-PCR negative, with and without symptoms. Based on three methods of determining cut-off, the optimum cut-off value of C4b was ≥ 116 ng/ml with the optimum sensitivity 80% and specificity 52.17% The mean area under the curve was 0.70 with 95% Confidence Interval (CI) of AUC 0.614–0.786. Likelihood ratio was determined to be + 1.67 with accuracy = 62.4%
Next, we determined the level of C5b-9 (membrane attack complex) which is the terminal complement pathway effector to form cytotoxic pores on the surface of pathogens and it is associated with Covid-19 patients [17]. Figure 2A presents the mean comparison of C5b-9 between Covid-19 cases and healthy controls. The levels of C5b-9 were not statistically different in these groups.
Evaluation of plasma levels of (A) C5b-9 and (B) C1-INH. (A) Mean comparison of plasma C5b-9 levels (ng/ml) between Covid19 cases (1) and controls (0): Mean C5b-9 levels with 95% CI in cases and controls were 257.90 (223.47–292.32) and 235.10 (205.23–264.95) respectively, and were not significantly different (t-stat = 1.01; p-value = 0.3144). (B) Mean comparison of plasma C1-INH levels (ng/ml) between Covid19 cases (1) and controls (0): Mean C1-INH levels with 95% CI in cases and controls were 510.09 (383.94–636.24) and 1124.20 (959.10 − 1289.30) respectively, and were significantly lower in cases (t-stat=-5.97; p-value = 0.0001)
The regulation complement activation was assessed by measuring C1-INH and the mean values of C1-INH in Covid-19 cases were significantly lower than controls (Fig. 2B).
Further, to understand early pathogenesis of Covid-19, we measured IL-6 levels as the inflammatory cytokines as they are associated with ICU Covid-19 patients [18, 19]. The mean levels of IL-6 in Covid-19 cases were significantly higher than controls (Fig. 3A) D-dimer, a marker of coagulopathy has been found to be increased during Covid-19 progression [20], thus we also measured it in Covid-19 cases. Significantly increased levels of D-dimer were observed in Covid-19 cases than controls (Fig. 3B).
Evaluation of plasma levels of IL6 ( A) and (B) D-dimer. (A) Mean comparison of IL-6 levels (pg/ml) between Covid19 cases (1) and controls (0): Mean IL6 levels with 95% CI in cases and controls were 44.68 (26.08–63.28) and 3.07 (2.26–3.89) respectively and were significantly higher in cases (t-stat = 4.52; p-value = 0.0001). (B) Mean comparison of D-dimer levels (ng/ml) between Covid19 cases (1) and controls (0): Mean D-dimer levels with 95% CI in cases and controls were 2.27 (0.94–3.59) and 0.365 (0.296–0.435) respectively, and were significantly higher in cases (t-stat = 2.90; p-value = 0.0048)
Discussion
The immune-driven pathologies have been observed in severe cases of SARS-CoV-2 infections with proven contribution of complement activation in its pathogenesis [6]. The spike protein of SARS-CoV-2 has been identified as the activator of the complement system [21]. Multiple studies have reported increased complement activation products in the plasma and/or their deposition in target organs of patients with Covid-19 infection [6, 22, 23].
Thus, we evaluated plasma levels of complement fragments like C4b and C5b-9 in the mild and stable patients suffering from Covid-19 infection and who were RT-PCR positive. The C4b levels in symptomatic RT-PCR negative cases were also evaluated. Asymptomatic RT-PCR negative participants were taken as controls. Our findings reported significantly higher levels of C4b in Covid-19 patients. We speculate that the complement system in Covid-19 infection may be over-activated and hence the increased levels of C4b. Thus, the C4b complement fragment being the first cleaved product of activated C4, may act as an index to mark the activation of complement by Lectin Pathway (LP) or Classical Pathway (CP). Earlier a study on pulmonary tuberculosis had used serum levels of C4b as the marker of complement activation in the early stage of LP activation [24]. Also, a recent study reports the binding of C3b and C4b with recombinant SAR CoV2 proteins (S or N) [10]. Hence, in Covid-19 infection too, plasma C4b levels may be used as a marker of complement activation, ensuring the presence of virus in the host. Further, we found significantly increased C4b levels in symptomatic cases also, who tested negative by RT-PCR. It is possible that the viral load in the symptomatic RT-PCR negative group might have been very low to be detected by RT-PCR test; however, this viral load was sufficient to activate the host’s complement system thereby producing C4b fragments which were measured. Importantly, whereas low sensitivity of RT-PCR test has not been able to detect virus in mild to moderate infection due to low titer [5, 10], evaluation of C4b levels may help to mark the presence of virus even at its very low titer. Similar observation has been reported in HIV patients [9] who had viremia which was undetectable with conventional assays [25,26,27,28,29,30]. However, the plasma levels of complement Factor I and C4b peptides had strong predictive value to detect viremia in these individuals [9]. In our study, the C4b levels in symptomatic but RT-PCR negative cases were significantly different from asymptomatic RT-PCR negative controls affirming the sensitivity and specificity of C4b measurement. Hence, the plasma C4b levels with a cutoff value ≥ 116ng/ml for Covid-19 cases relative to controls, as determined in our study, with the optimum sensitivity and specificity 80% and 52% respectively, may be used to predict viremia even at low titer. It is even more important in the initial phase of infection, which is presented with mild symptoms [12,13,14,15] and when 54% of Covid-19 patients may have an initial false-negative RT-PCR (very low certainty of evidence) [31]. Hence, to limit the transmission of Covid-19 infection, it is critical that the cases presented with mild symptoms should be detected and not be labeled as negative.
Therefore, we speculate that C4b, if studied on large number of subjects of all age groups, individuals with comorbid conditions and ICU patients, can be an adjunct marker for diagnosing symptomatic patients tested negative by RT-PCR.
Further, C5b-9 levels have been reported as an index of severity in Covid-19 infection as C5b-9 also has a role in neutrophil activation and inflammation that leads to endothelial damage [22, 23]. Our findings on C5b-9 levels showed no significant increase in cases than controls as the patients had mild to moderate clinical presentation and were stable when the blood samples were collected.
The activation of complement pathways is regulated by many proteins such as C1-INH, C4BP (C4b-binding protein), MAP-1 (MBL/ficolin/CL-associated protein-1) and sMAP (small MBL-associated protein) [32]. Also, it has been reported that a pathogen specific mechanism in SARS-CoV2 directly depletes C1-INH, which is responsible for several systemic manifestations exhibited by Covid-19 patients [33]. Activation of the complement system leads to consumption of C1-INH leading to decrease in its serum levels [34, 35]. We observed significantly lower levels of C1-INH inCovid-19 patients as compared to controls, thus suggesting over activation of the complement system in Covid-19 infection leading to consumption and depletion of C1-INH.
Coagulopathy has been a striking feature in Covid-19 infection. It has been scientifically proven that there is a high degree of crosstalk between complement and coagulation pathways [36,37,38]. It means that over activated complement is associated with activated coagulation process also [7]. In our study D-dimers levels were found to be significantly increased in cases than in controls indicating increased coagulopathy. Also, significantly increased levels of IL6 found in our study among cases further confirms proinflammatory state in Covid-19 infection which is associated with over activated complement system [39].
An earlier study on viral infections found that complement C4 has an antiviral effect independent of downstream complement activation and prevents viral infection through capsid inactivation [8]. Also, the plasma levels of complement Factor I and C4b peptides are associated with HIV suppression [9]. Further research is needed to ascertain if C4 has any antiviral activity against Covid-19 also.
Conclusions
The results of our study suggest that the evaluation of plasma C4b if studied on large number of subjects of all age groups, individuals with comorbid conditions and ICU patients, can be an adjunct marker for diagnosing symptomatic patients tested negative by RT-PCR.
At last, it is important to note that sampling errors are minimal in taking blood samples as compared to oropharyngeal and nasopharyngeal swabs which needs skilled hands. It will always be safer to have a marker in blood than in other biological samples.
Availability of data & Material
Yes.
Code Availability
Not applicable.
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. DOI:https://doi.org/10.1056/NEJMoa2001017.
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–9. https://doi.org/10.1038/s41586-020-2008-3.
Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2020;(December). https://doi.org/10.1038/ s41579-020-00459-7.
Kortela E, Kirjavainen V, Ahava MJ, Jokiranta ST, But A, Lindahl A, et al. Real-life clinical sensitivity of SARS-CoV-2 RT-PCR test in symptomatic patients. PLoS ONE. 2021;16(5 May):1–19. https://doi.org/10.1371/journal.pone.0251661.
Woloshin S, Patel N, Kesselheim AS. False Negative Tests for SARS-CoV-2 Infection — Challenges and Implications. N Engl J Med. 2020 Aug;383(6):e38. DOI:https://doi.org/10.1056/NEJMp2015897.
Java A, Apicelli AJ, Kathryn Liszewski M, Coler-Reilly A, Atkinson JP, Kim AHJ, et al. The complement system in COVID-19: Friend and foe? JCI Insight. 2020 Aug; 5(15). https://dx.doi.org/10.1172%2Fjci.insight.140711.
Lo MW, Kemper C, Woodruff TM. COVID-19: Complement, Coagulation, and Collateral Damage. J Immunol. 2020;205(6):1488–95.
Maria Bottermann S, Foss SL, Caddy. et al. Inger Sandlie, Jan Terje Andersen LCJ. Complement C4 Prevents Viral Infection through Capsid Inactivation.pdf.
Wu B, Ouyang Z, Lyon CJ, Zhang W, Clift T, Bone CR, et al. Plasma Levels of Complement Factor i and C4b Peptides Are Associated with HIV Suppression. ACS Infect Dis. 2017;3(12):880–5.
Ali YM, Ferrari M, Lynch NJ, Yaseen S, Dudler T, Gragerov S, et al. Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front Immunol. 2021;12(July):1–8.
Glioma H, Incidentaloma A. C or r e sp ondence Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. 2021.
Levine-Tiefenbrun M, Yelin I, Uriel H, Kuint J, Schreiber L, Herzel E, et al. Association of COVID-19 RT-qPCR test false-negative rate with patient age, sex and time since diagnosis. medRxiv. 2020;2020.10.30.20222935.
He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–5.
Lurie N. Rethinking Covid-19 Test Sensitivity - A Strategy for Containment. N Engl J Med. 2020;120(1):1969–73.
Cevik M, Marcus J, Buckee C, Smith T. SARS-CoV-2 Transmission Dynamics Should Inform Policy. SSRN Electron J. 2020;1–19.
Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;Vol. 27:790–2.
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020 Jun;220(January):1–13.
Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(January):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Lavillegrand JR, Garnier M, Spaeth A, Mario N, Hariri G, Pilon A, Berti E, Fieux F, Thietart S, Urbina T, Turpin M. Elevated plasma IL-6 and CRP levels are associated with adverse clinical outcomes and death in critically ill SARS-CoV-2 patients: inflammatory response of SARS-CoV-2 patients. Ann Intensiv Care. 2021 Dec;11(1):1–0. https://doi.org/10.1186/s13613-021-00879-5.
Fletcher-sandersjöö A, Bellander B. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company ’ s public news and information. 2020;(January).
Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D Inhibition. Blood. 2020;136(18):2080–9.
Piepoli MF. Confronting the reality of COVID. Eur J Prev Cardiol. 2020;27(8):787–8.
Cugno M, Meroni PL, Gualtierotti R, Griffini S, Grovetti E, Torri A, et al. Complement activation in patients with COVID-19: A novel therapeutic target. J Allergy Clin Immunol. 2020 Jul;146(1):215–7.
Wang C, Li YY, Li X, Wei LL, Yang XY, Xu DD, et al. Serum complement C4b, fibronectin, and prolidase are associated with the pathological changes of pulmonary tuberculosis. BMC Infect Dis. 2014;14(1):1–9.
Okulicz JF, Marconi VC, Landrum ML, Wegner S, Weintrob A, Ganesan A, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US department of defense HIV natural history study. J Infect Dis. 2009;Vol. 200:1714–23.
Hubert JB, Burgard M, Dussaix E, Tamalet C, Deveau C, Le Chenadec J, et al. Natural history of serum HIV-1 RNA levels in 330 patients with a known date of infection. Aids. 2000;14(2):123–31.
Deeks SG, Walker BD. Human Immunodeficiency Virus Controllers: Mechanisms of Durable Virus Control in the Absence of Antiretroviral Therapy. Vol. 27, Immunity. 2007. p. 406–16.
Grabar S, Selinger-Leneman H, Abgrall S, Pialoux G, Weiss L, Costagliola D. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. Aids. 2009;23(9):1163–9.
Hunt PW. Natural control of HIV-1 replication and long-term nonprogression: Overlapping but distinct phenotypes. J Infect Dis. 2009;Vol. 200:1636–8.
Saag M, Deeks SG. How do HIV elite controllers do what they do? Clin Infect Dis. 2010;51(2):239–41.
Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, Zambrano-Achig P, Campo R, Del, Ciapponi A, et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS ONE. 2020;15(12 December):1–19.
CQ S, JD L. D R. Protection of host cells by complement regulators. Immunol Rev. 2016;118(24):6072–8. https://doi.org/10.1111/imr.12475.
Thompson DC, Barbu MG, Beiu C, Popa LG, Mihai MM, Berteanu M, et al. The Impact of COVID-19 Pandemic on Long-Term Care Facilities Worldwide: An Overview on International Issues. BioMed Res Int. 2020;2020.
Zuraw BL, Altman LC. Acute consumption of C1 inhibitor in a patient with acquired C1-inhibitor deficiency syndrome. J Allergy Clin Immunol. 1991;88(6):908–18.
Bonanome A, Grundy SM. Acquired C1-inhibitor deficiency associated with antiidiotypic antibody to monoclonal immunoglobulins. N Engl J Med. 1988;1244–8.
Dobó J, Schroeder V, Jenny L, Cervenak L, Závodszky P, Gál P. Multiple roles of complement MASP-1 at the interface of innate immune response and coagulation. Mol Immunol. 2014;61(2):69–78.
Gulla KC, Gupta K, Krarup A, Gal P, Schwaeble WJ, Sim RB, et al. Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology. 2010;129(4):482–95.
Krarup A, Gulla KC, Gál P, Hajela K, Sim RB. The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim Biophys Acta - Proteins Proteomics. 2008;1784(9):1294–300.
Zhang J, Hao Y, Ou W, Ming F, Liang G, Qian Y, et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: a cohort study. J Transl Med. 2020;18(1):1–8.
Funding
This work was supported by All India Institute of Medical Sciences, Patna (Grant number I-6/529).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest/Competing Interest
None.
Ethics approval
Yes.
Consent to participate
Yes.
Consent to publish
Yes.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumari, B., Hajela, K., Ali, A. et al. Evaluation of C4b as an adjunct marker in symptomatic RT-PCR negative Covid-19 cases. Ind J Clin Biochem 38, 102–109 (2023). https://doi.org/10.1007/s12291-022-01033-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-022-01033-z