Abstract
Convalescent plasma is currently being used in the treatment of COVID-19. Recommendations regarding use convalescent plasma in COVID-19 requires systematic summaries of available evidence. We searched the databases Medline, Embase, Cochrane CENTRAL, Epistomonikos, Medrxiv and Biorxiv. Title/abstract screening, full text screening and data abstraction were carried out in duplicate by two reviewers. Pooled effect sizes and 95% confidence intervals were calculated using random effects meta-analysis. GRADE tool was used to rate the certainty of evidence. Twenty two studies were found eligible for inclusion: nine randomized controlled trials and thirteen cohort studies. Low certainty evidence from eight RCTs showed inconclusive effects of convalescent plasma on mortality at 28 days (OR 0.85, 95% CI 0.61 to 1.18). Low certainty evidence from thirteen cohort studies showed a reduction in mortality at 28 days (OR 0.66, 95% CI 0.53 to 0.82). The pooled OR for clinical improvement was 1.07 (95% CI 0.86 to 1.34) representing low certainty evidence. Evidence from three RCTs showed inconclusive effect of CP on the need for mechanical ventilation (OR 1.20, 95% CI 0.72 to 1.98). Four cohort studies reporting unadjusted estimates suggested a reduction in the need for mechanical ventilation with convalescent plasma (OR 0.80 95% CI 0.71 to 0.91, low certainty). Pooled estimates from 2 RCTs showed inconclusive effects of convalescent plasma on the proportion of patients with nondetectable levels of virus in nasopharyngeal specimens on day 3 (OR 3.62, 95% CI 0.43, 30.49, very low-quality evidence). The present review reports uncertain estimates on the efficacy of convalescent plasma in the treatment of COVID-19. There is low certainty evidence of a possible reduction in mortality and mechanical ventilation, a faster viral clearance and the absence of any serious adverse events. However, its efficacy for these outcomes requires evidence from good quality and adequately powered randomized controlled trials.
Similar content being viewed by others
Introduction
The worldwide spread of COVID-19, the illness caused by SARS-CoV-2, poses an enormous threat to human health, and is a cause of major social and economic crises worldwide. As of January 2021, COVID-19 has resulted in more than 2 million deaths worldwide (1), and its transmission continues unabated in many parts of the globe.
The massive potential for adverse health consequences from this pandemic has led to a desperate need for interventions that can reduce the resulting morbidity and mortality. Research is ongoing to develop vaccines and identify therapeutics for COVID-19, including repurposing of medications [2]. A globally implemented, safe mass vaccination programme seems to be the only long term solution; [3]. Interventions with evidence of reducing morbidity and mortality from this disease are urgently required in order to form the bridge to the endgame of mass vaccination.
However, the use of medications without proven effectiveness may result in avoidable harm to patients, detract investment in other resources and erode public trust in the medical community [4, 5].
Convalescent plasma (CP) is currently being used in the treatment of COVID-19 with the rationale that it contains antibodies that potentially neutralize the virus and thus prevent the inflammatory cascade that ensues. It has been used historically for the treatment of infectious diseases and there is also some evidence of its usefulness in the treatment of other coronavirus infections like SARS and MERS [6, 7].
Recommendation regarding use convalescent plasma in COVID-19 requires systematic summaries of available evidence. At a time when research is being produced at an unprecedented pace, it is necessary to rigorously appraise the evidence to distinguish the trustworthy from the untrustworthy. Moreover, in the context of COVID-19, where the best evidence is constantly changing, it is required that we have the most updated summaries available for practice. Therefore, we conducted a systematic review and meta-analysis to study the efficacy and safety of convalescent plasma in patients with COVID-19.
Methodology
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Meta-analysis of observational studies in epidemiology (MOOSE) statements were adhered to in the present report [8, 9]. We developed a protocol before beginning this systematic review. The protocol for this review was registered in PROSPERO, with the registration number CRD42020227417.
Inclusion Criteria
The following inclusion criteria were used for eligibility:
Type of participants We included studies on patients with severe and non-severe COVID-19.
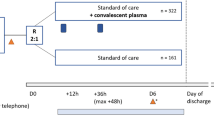
Type of intervention We included studies assessing intravenous convalescent or hyperimmune plasma as an intervention in addition to standard of care
Type of outcomes We included studies reporting the following outcomes:
Primary outcome: Overall mortality
Secondary outcomes:
Clinical recovery
Rate of ICU admission
Length of ICU stay
Length of hospital stay
Need for mechanical ventilation
Viral clearance
Adverse events: Intravascular volume overload related complications and transfusion-related acute lung injury, allergy or anaphylaxis, and any other patient-important safety outcomes that study reports
Type of Studies We included randomized controlled trials, cohort studies and case series that compared the use of convalescent plasma to treatment without convalescent plasma and reported on at least one of our outcomes of interest. We excluded case series in which all patients, or no patients, received convalescent plasma.
Data sources and Searches
We searched in the following databases: Medline (Ovid), Embase, Cochrane CENTRAL, Epistomonikos and ran a PubMed search for studies not yet indexed in Medline. We reviewed reference lists of all included studies and relevant systematic reviews for additional references. We searched medRxiv and biorxiv for any relevant pre-print previews.
No language restriction was imposed. The search strategy implemented is provided in Appendix 1. The search was updated till 15th January 2021.
Study Selection
Pairs of reviewers independently screened titles and abstracts, and reviewed the full texts of potential eligible studies to determine the final eligible studies. Disagreements were resolved by discussion or by referring to a third reviewer.
Data Extraction
Pairs of reviewers abstracted data. We abstracted surname of the first author, year of publication, country, region and hospital, population, interventions, and outcomes. For cohort studies, we also abstracted the method of adjustment used and covariates adjusted for in the analysis. Disagreements were resolved by discussion or, if necessary, by a third reviewer.
Risk of Bias Assessment
We assessed the risk of bias in randomized trials using the modified Cochrane tool that includes sequence generation, allocation sequence concealment, blinding, and missing outcome data. Each criterion was judged as definitely or probably low risk of bias, or probably or definitely high risk of bias [8]. We assessed risk of bias in cohort studies using the New Castle Ottawa scale for Cohort studies [9]. Two review authors independently assessed the study risk of bias with disagreements resolved by involving a third reviewer.
Data Synthesis or Analysis
We calculated pooled odds ratio (OR) and 95% confidence intervals (95% CI) for dichotomous outcomes. We used adjusted estimates from studies wherever reported, and used the random-effects models to pool study data. We used DerSimonian and Laird inverse variance random-effect models to pool adjusted odds ratios (ORs). We carried out all statistical analyses using Review Manager 5.3. We assessed heterogeneity in the meta-analyses by visual inspection of the forest plot and by the I2 statistic.
GRADE Assessment of the Overall Quality in the Body of Evidence by Outcome
We used the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) methodology to rate the certainty in evidence for each outcome as high, moderate, low, or very low [10]. The assessment included judgments addressing risk of bias, imprecision, inconsistency, indirectness, and publication bias.
We summarized the evidence both narratively and in GRADE evidence profiles.
Results
Study Selection
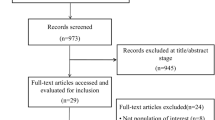
Our search yielded 3993 titles and abstracts - all were identified from the electronic database search. We excluded 3935 articles based on a review of the title and abstract, leaving 58 articles for full review. Of these, 36 were excluded- 33 for having an inappropriate study design, two for having inappropriate comparison, one for including a duplicate population. Twenty two studies were found eligible on full text screening. Nine of these were pre-print articles that had not been peer reviewed [12,13,14,15,16, 20, 24, 28, 32]. These 22 studies were included in the systematic review and meta-analyses. [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] (Fig. 1).
Study Characteristics and Estimates Reported
The studies included were nine randomized controlled trials (1254 patients) [11,12,13,14,15,16, 26,27,28] and thirteen cohort studies (8368 patients) [16,17,18,19,20,21,22,23,24, 29,30,31,32]. All studies included patients hospitalized with COVID-19 (Tables 1 and 2). Most studies included patients with severe COVID-19 except one study that included moderately ill patients [12].
Risk of Bias Assessment
All trials were assessed to have low risk of bias for adequate random sequence generation and allocation concealment. Risk of bias for selective outcome reporting and missing outcome data was assessed to be low as most studies reported important outcomes and very less loss to follow up. However, only two trials were blinded and four were stopped early [11, 13, 14, 27]. Three trials reported stopping early because the epidemic had considerably diminished and the trial saw low enrolment [11, 14, 27]; while one reported stopping early since a high proportion of patients were found to have SARS-CoV-2 antibodies at baseline [13]. All trials were also assessed to be high risk of bias due to imbalance of co-interventions amongst the two arms.
For all outcomes in the cohort studies, risk of bias was low for selection of exposed and non-exposed population and assessment of exposure. All cohort studies were assessed to have low risk of bias from outcome being present at the start of the study. However, adequate adjustment and assessment of prognostic factors were not carried out by three studies. Follow up was adequate for all outcomes in the cohort studies; however, no study documented similar co-interventions in both groups (Tables 3 and 4).
Pooled Effects of Convalescent Plasma on Safety and Efficacy Outcomes
Mortality
Low certainty evidence from 8 RCTs (n = 1374) (11−15, 26-28) showed inconclusive effects of convalescent plasma on mortality at 28 days with a possible but uncertain reduction in mortality (OR 0.85, 95% CI 0.61 to 1.18, I2=0%) (Fig. 2 and Table 5).
Low certainty evidence from 13 cohort studies [17,18,19,20,21,22,23,24,25, 29,30,31,32] (n=8368) showed a reduction in mortality at 28 days (OR 0.66, 95% CI 0.53 to 0.82, I2=26%). (Fig. 3 and Table 5).
Clinical Improvement
Two RCTs reported ordinal outcomes relating to extent of clinical improvement at 30 days and one RCT at 14 days. The pooled OR for clinical improvement (522 patients) [11, 13, 26] was OR 1.07 (95% CI 0.86 to 1.34) representing low certainty evidence (Fig. 4 and Table 5).
Duration of Hospital Stay
Evidence from 3 RCTs and one cohort study [12, 15, 25, 26] suggested uncertain effect of convalescent plasma on decreasing length of hospital stay (MD 0.12, 95% CI –1.69 to 1.93) (Fig. 5 and Table 5).
Need for Mechanical Ventilation
Evidence from three RCTs (n=522) [12, 16, 26] showed inconclusive effect of CP on need for mechanical ventilation (OR 1.20, 95% CI 0.72 to 1.98, I2=0%, low certainty). However, four cohort studies reporting unadjusted estimates suggested a reduction in the need for mechanical ventilation with convalescent plasma (OR 0.80 95% CI 0.71 to 0.91, I2=0%, low certainty) (Fig. 6 and Table 5).
Viral Clearance
One RCT (n=522) [12] showed higher proportion of patients with undetectable virus on day 7 (OR 1.52, 95% CI 1.06, 2.20).
Pooled estimates from 2 RCTs (n = 609) [11, 12] showed inconclusive effects of convalescent plasma on the proportion of patients with nondetectable levels of virus in nasopharyngeal specimens on day 3 (OR 3.62, 95% CI 0.43, 30.49, very low-quality evidence) (Fig. 7 and Table 5).
There were very few adverse events reported in all included studies, with most trials and cohort studies not reporting any adverse events.
Discussion
The results of our meta-analysis show that there is very low to low certainty evidence to suggest that CP does not reduce mortality, hospital stay, need for mechanical ventilation or improvement in clinical status. Based on a single study, whether CP improves viral clearance is not clear.
The theory behind using CP for treatment goes back to the early days of antimicrobial therapy when it was used for pneumococcal pneumonia [6]. More recently, it has been used in the treatment for various viral diseases like SARS, MERS and Ebola [7]. The rationale is that antibodies in CP have the capacity to work in two different ways: first is the potential to act on the microbes causing the infection, and the second is that they can exert immunomodulatory therapy, thus boosting the immunity of the patient.
The included studies demonstrated a very good safety profile for CP. There is a theoretical possibility of antibody dependent enhancement [33], though there are no reports of this from trials.
However, the use of convalescent plasma as therapy for COVID-19 is not without challenges. Only limited quantities of plasma can be obtained at a time and its collection consumes resources. There is considerable difference in the titres, antibody response and type of antibodies amongst people. Besides this, though most studies did not report many adverse events, there are safety issues related to transfusion such as acute lung injury, transfusion related circulatory overload and anaphylaxis that need to be managed at times [34]. Risk of transmission of blood borne infections is also a potential threat to recipients of CP.
In March 2020, the US FDA approved convalescent plasma as an investigational new drug in the expanded access program. The unadjusted analysis of data from the EAP, though not comparing CP with no CP, suggested that treatment given early after diagnosis (within 3 days) is associated with lower 7-day and 30-day mortality, and transfusion of convalescent plasma with high SARS-CoV-2 IgG antibody levels compared to medium or low IgG levels was furthermore associated with lower mortality. This could be an explanation as to why other studies investigating convalescent plasma may not be able to detect a clinically relevant difference, although these findings should be confirmed in RCTs before it can be translated into practice [35].
To the best of our knowledge, there is no previous systematic review that has included all the studies included in the present review. Some previous systematic reviews and meta-analyses have evaluated the efficacy of CP in the treatment of COVID-19. Chai et al in a Cochrane review that included two trials and eight NRSIs, concluded that there is uncertain evidence of the effect of CP on mortality (risk ratio (RR) 0.55, 95% CI 0.22 to 1.34, low-certainty evidence) and clinical improvement (RR 0.98, 95% CI 0.30 to 3.19, low-certainty evidence) [36]. This conclusion is consistent with what the present study found. The systematic review by Shao et al evaluated the effect of convalescent blood products (CBPs) for patients with severe acute respiratory infections of all viral aetiologies, and concluded that the all-cause mortality in the RCTs showed no difference between the interventional group and the control group (OR 0.82; 95% CI 0.57 to 1.19; P = 0.30). Using CBPs earlier, compared with using CBPs later, was associated with a significant reduction in all-cause mortality (OR 0.18; 95% CI 0.08-0.40; P < 0.0001) [37]. Sarkar et al identified two RCTs and five cohort in their systematic review on role of CP in COVID-19. They concluded that there is very low- to low quality evidence that CP reduces mortality (OR 0.44, 95% CI 0.25 to 0.77) from seven studies and increases clinical improvement (OR 2.06, 95% CI 0.8 to 4.9) from five studies [38]. The review by Devasenapathy et al provided indirect evidence of the role of CP from studies on SARS, MERS, Influenza and Ebola. However, they reported very low-quality evidence that raised the possibility that CP has minimal or no benefit in the treatment of COVID-19 and low-quality evidence that it does not cause serious adverse events [7].
The present systematic review incorporates a robust search strategy with a comprehensive search of three major databases as well as preprint servers. Screening of titles, abstracts and full texts was done independently by two reviews in duplicate, as was the data abstraction including risk of bias assessments. Lastly, we used the GRADE methodology to rate the certainty in the evidence, thus enabling us to pay close attention to methodological issues like inconsistency, imprecision and risk of bias.
The limitations of the present review are largely due to the limitations of the included primary studies, most of which yielded imprecise estimates. Imprecision persisted for most outcomes in the meta-analyses since evidence from large RCTs are not yet available. Though we used the adjusted estimates wherever reported, many studies failed to report adjusted estimates and were assessed as having serious risk of bias due to confounding. Another consideration is that this review includes evidence from pre-print articles, the quality of which has not been peer-reviewed and the results of which are subject to change following the peer review process. The number of included studies were few, precluding the possibility of subgroup analyses to explore the heterogeneity observed in the meta-analyses of some outcomes. However, the difference in severity of patients included amongst trials could possibly explain the heterogeneity. For instance, Agarwal et al [12] enrolled patients that were less severe than the other trials in the meta-analyses. Clinical improvement scales were different between the various included trials. Differences in the interventions with respect to titres, volume, etc could also be a source of heterogeneity. Clinical heterogeneity is evident from the different inclusion criteria used by studies. There is limited knowledge so far with regard to what level of antibody titre is protective and three of the included studies did not measure antibody titres of donors [19, 21, 25]. There is very limited reporting of adverse events in the included studies. Many studies excluded patients with moderate to severe ARDS and this precluded studying the effect of severity of disease on the efficacy of CP.
Uncertainty remains regarding the role of convalescent plasma in the treatment of COVID-19. There is low certainty evidence of a possible reduction in mortality and mechanical ventilation, a faster viral clearance and the absence of any serious adverse events. However, its efficacy for these outcomes requires evidence from good quality and adequately powered randomized controlled trials.
References
COVID-19 Map [Internet]. Johns Hopkins coronavirus resource center. [cited 2020 Sep 30]. Available from: https://coronavirus.jhu.edu/map.html
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB (2020) Pharmacologic treatments for coronavirus disease 2019 COVID-19. A review. JAMA. https://doi.org/10.1001/jama.2020.6019
O’Callaghan KP, Blatz AM, Offit PA (2020) Developing a SARS-CoV-2 vaccine at warp speed. JAMA 324(5):437–438. https://doi.org/10.1001/jama.2020.12190 (PMID: 32628244)
Goodman JL, Borio L (2020) Finding effective treatments for COVID-19: scientific integrity and public confidence in a time of crisis. JAMA. https://doi.org/10.1001/jama.2020.6434
Juurlink DN (2020) Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ Can Med Assoc J J Assoc Medicale Can 192(17):E450-3
Xi Y (2020) Convalescent plasma therapy for COVID-19: a tried-and-true old strategy? Signal Transduct Target Ther 5(1):203. https://doi.org/10.1038/s41392-020-00310-8 (PMID: 32934211)
Devasenapathy N, Ye Z, Loeb M, Fang F, Najafabadi BT, Xiao Y et al (2020) Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis. CMAJ 192(27):E745–E755. https://doi.org/10.1503/cmaj.200642 (Epub 2020 May 22 PMID: 32444482)
Guyatt GH, Busse JW. (2020) Modification of cochrane tool to assess risk of bias in randomized trials. [Internet]. [cited 2020 Sep 30]. Available from: https://www.evidencepartners.com/resources/methodological-resources/
Busse JW, Guyatt GH. (2020) Tool to assess risk of bias in cohort studies. [Internet]. [cited 2020 Sep 30]. Available from https://www.evidencepartners.com/resources/methodological-resources/
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–6 (Epub 2008/04/26)
Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J et al (2020) Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 324(5):460–70
Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, et al. (2020) Convalescent plasma in the management of moderate COVID-19 in India: An open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial). medRxiv. 10;09.03.20187252
Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN et al (2020) Convalescent plasma for COVID-19 A randomized clinical trial. medRxiv 07(01):20139857
Avendano-Sola C, Ramos-Martinez A, Munez-Rubio E, Ruiz-Antoran B, Malo de Molina R, Torres F, et al. (2020) Convalescent Plasma for COVID-19: A multicenter, randomized clinical trial. medRxiv. 1(08):26-20182444
Bajpai M, Kumar S, Maheshwari A, Chabra K, Kale P, Gupta A, et al. (2020) Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients A Pilot Randomized Controlled Trial. medRxiv. 27(10): 25-20219337
AlQahtani M, Abdulrahman A, Almadani A, Alali SY, Al Zamrooni AM, Hejab AH, et al. (2020) Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. medRxiv. 1(11): 02-20224303
Rasheed AM, Ftak DF, Hashim HA, Maulood MF, Kabah KK, Almusawi YA, et al. (2020) The therapeutic effectiveness of Convalescent plasma therapy on treating COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. medRxiv. 1(06): 24-20121905
Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S et al (2020) Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus Apheresis Sci: Off J World Apheresis Assoc: Off J Eur Soc Haemapheresis. https://doi.org/10.1016/j.transci.2020.102875
Omrani AS, Zaqout A, Baiou A, Daghfal J, Elkum N, Alattar RA et al (2020) Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: a preliminary report. J Med Virol 93(3):1678–1686
Rogers R, Shehadeh F, Mylona E, Rich J, Neill M, Touzard-Romo F, et al. (2020) Convalescent plasma for patients with severe COVID-19: a matched cohort study. medRxiv. 1(08): 18-20177402
Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. (2020) Treatment of COVID-19 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. The American journal of pathology [Internet].; Available from: http://www.epistemonikos.org/documents/29ee31cf41f2718027546e3b0493e08db5f18c71
Xia X, Li K, Wu L, Wang Z, Zhu M, Huang B et al (2020) Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood 136(6):755–9
Liu STH, Lin H-M, Baine I, Wajnberg A, Gumprecht JP, Rahman F et al (2020) Convalescent plasma treatment of severe COVID-19 a propensity score-matched control study. Nat Med 26(11):1708–1713
Salazar MR, González SE, Regairaz L, Ferrando NS, González Martínez VV, Carrera Ramos PM, et al. (2020) Effect of convalescent plasma on mortality in patients with Covid-19 pneumonia. medRxiv. 1(10): 08-20202606
Altuntas F, Ata N, Yigenoglu TN, Bascı S, Dal MS, Korkmaz S et al (2020) Convalescent plasma therapy in patients with COVID-19. Transfus Apher Sci 19:102955–102955
Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C et al (2020) A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. https://doi.org/10.1056/NEJMoa2031304
Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V et al (2021) Early High-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. https://doi.org/10.1056/NEJMoa2033700
Ray Y, Paul SR, Bandopadhyay P, D’Rozario R, Sarif J, Lahiri A, et al. (2020) Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. 111(25): 20237883
Hegerova L, Gooley TA, Sweerus KA, Maree C, Bailey N, Bailey M et al (2020) Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood 136(6):759–762. https://doi.org/10.1182/blood.2020006964
Jiang W, Li W, Xiong L, Wu Q, Wu J, He B et al (2020) Clinical efficacy of convalescent plasma therapy on treating COVID-19 patients: Evidence from matched study and a meta-analysis. Clin Transl Med 10(8):e259. https://doi.org/10.1002/ctm2.259
Alsharidah S, Ayed M, Ameen RM, Alhuraish F, Rouheldeen NA, Alshammari FR et al (2020) COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: a multicenter interventional study. Int J Infect Dis 4(103):439–446. https://doi.org/10.1016/j.ijid.2020.11.198
Yoon HA, Bartash R, Gendlina I, Rivera J, Nakouzi A, Bortz RH et al (2020) Treatment of severe COVID-19 with convalescent plasma in the Bronx NYC. medRxiv. https://doi.org/10.1101/2020.12.02.20242909
Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C et al (2020) A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 584(7821):353–363. https://doi.org/10.1038/s41586-020-2538-8
Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA et al (2020) Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest 130(9):4791–4797. https://doi.org/10.1172/JCI140200
Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. (2020) Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial Three-month experience. medRxiv. 1(08): 12-20169359
Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, Doree C, et al. (2020) Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev 12(10): CD013600
Shao S, Wang Y, Kang H, Tong Z (2020) Effect of convalescent blood products for patients with severe acute respiratory infections of viral etiology: a systematic review and meta-analysis. Int J Infect Dis S1201–9712(20):32159–7. https://doi.org/10.1016/j.ijid.2020.09.1443
Sarkar S, Soni KD, Khanna P (2020) Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol. https://doi.org/10.1002/jmv.26408
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prasad, M., Seth, T. & Elavarasi, A. Efficacy and Safety of Convalescent Plasma for COVID-19: A Systematic Review and Meta-analysis. Indian J Hematol Blood Transfus 37, 347–365 (2021). https://doi.org/10.1007/s12288-021-01417-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-021-01417-w