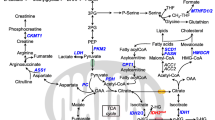
Abstract
Leukemia is one of the most aggressive hematological malignancies. Leukemia stem cells account for the poor prognosis and relapse of the disease. Decades of investigations have been performed to figure out how to eradicate the leukemia stem cells. It has also been known that cancer cells especially solid cancer cells use energy differently than most of the cell types. The same thing happens to leukemia. Since there are metabolic differences between the hematopoietic stem cells and their immediate descendants, we aim at manipulating the energy sources with which that could have an effect on leukemia stem cells while sparing the normal blood cells. In this review we summarize the metabolic characteristics of distinct leukemias such as acute myeloid leukemia, chronic myeloid leukemia, T cell lymphoblastic leukemia, B-cell lymphoblastic leukemia, chronic lymphocytic leukemia and other leukemia associated hematological malignancies such as multiple myeloma and myelodysplastic syndrome. A better understanding of the metabolic profiles in distinct leukemias might provide novel perspectives and shed light on novel metabolic targeting strategies towards the clinical treatment of leukemias.

Similar content being viewed by others
Abbreviations
- AML:
-
Acute myeloid leukemia
- CML:
-
Chronic myeloid leukemia
- T-ALL:
-
T-cell lymphoblastic leukemia
- B-ALL:
-
B-cell lymphoblastic leukemia
- CLL:
-
Chronic lymphocytic leukemia
- MM:
-
Multiple myeloma
- MDS:
-
Myelodysplastic syndrome
- LSCs:
-
Leukemia stem cells
- OXPHOS:
-
Oxidative phosphorylation
- FAO:
-
Fatty acid oxidation
- PPP:
-
Pentose phosphate pathway
- Glut1:
-
Glucose transporter 1
- Ara-C:
-
Arabinofuranosyl cytidine
- 2-DG:
-
2-Deoxy-d-glucose
- RTKs:
-
Receptor tyrosine kinases
- ROS:
-
Reactive oxygen species
- CSCs:
-
Cancer stem cells
- PPARγ:
-
Proliferator-activated receptor gamma ligands
- CPT1:
-
Carnitine O-palmitoyltransferase I
- GAT:
-
Gonadal adipose tissue
- GA:
-
Glutaminase
- IDH1/2:
-
Isocitrate dehydrogenase 1 and 2
- PHGDH:
-
Phosphoglycerate dehydrogenas
- I-ASP:
-
l-Asparaginase
- GS:
-
Glutamine synthase
- ASNS:
-
Asparagine synthetase
- PRC2:
-
Polycomb repressive complex 2
References
Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y (2008) PML targeting eradicates quiescent leukaemia-initiating cells. Nature 453(7198):1072–1078
Holtz M, Forman SJ, Bhatia R (2007) Growth factor stimulation reduces residual quiescent chronic myelogenous leukemia progenitors remaining after imatinib treatment. Can Res 67(3):1113–1120
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11(2):85–95
Schulze A, Harris AL (2012) How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 491(7424):364–373
Boroughs LK, DeBerardinis RJ (2015) Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 17(4):351–359
Kruiswijk F, Labuschagne CF, Vousden KH (2015) p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol 16(7):393–405
Jeon SM, Chandel NS, Hay N (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485(7400):661–665
Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP (2017) Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 14(2):113
DeBerardinis RJ, Cheng T (2010) Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29(3):313–324
Kumazaki M, Shinohara H, Taniguchi K, Takai T, Kuranaga Y, Sugito N et al (2016) Perturbation of the Warburg effect increases the sensitivity of cancer cells to TRAIL-induced cell death. Exp Cell Res 347(1):133–142
Wang Y-H, Israelsen William J, Lee D, Yu Vionnie WC, Jeanson Nathaniel T, Clish Clary B et al (2014) Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell 158(6):1309–1323
Saito Y, Chapple RH, Lin A, Kitano A, Nakada D (2015) AMPK protects leukemia-initiating cells in myeloid leukemias from metabolic stress in the bone marrow. Cell Stem Cell 17(5):585–596
Chen WL, Wang JH, Zhao AH, Xu X, Wang YH, Chen TL et al (2014) A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 124(10):1645–1654
Larrue C, Saland E, Vergez F, Serhan N, Delabesse E, Mansat-De Mas V et al (2015) Antileukemic activity of 2-deoxy-d-glucose through inhibition of N-linked glycosylation in acute myeloid leukemia with FLT3-ITD or c-KIT mutations. Mol Cancer Ther 14(10):2364–2373
Song K, Li M, Xu X, Xuan LI, Huang G, Liu Q (2016) Resistance to chemotherapy is associated with altered glucose metabolism in acute myeloid leukemia. Oncol Lett 12(1):334–342
Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M et al (2013) BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12(3):329–341
Kishton RJ, Barnes CE, Nichols AG, Cohen S, Gerriets VA, Siska PJ et al (2016) AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-all cell stress and survival. Cell Metab 23(4):649–662
Liu T, Kishton RJ, Macintyre AN, Gerriets VA, Xiang H, Liu X et al (2014) Glucose transporter 1-mediated glucose uptake is limiting for B-cell acute lymphoblastic leukemia anabolic metabolism and resistance to apoptosis. Cell Death Dis 5(10):e1470
Martinez Marignac VL, Smith S, Toban N, Bazile M, Aloyz R (2013) Resistance to dasatinib in primary chronic lymphocytic leukemia lymphocytes involves AMPK-mediated energetic re-programming. Oncotarget 4(12):2550–2566
Gottschalk S, Anderson N, Hainz C, Eckhardt SG, Serkova NJ (2004) Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin Cancer Res 10(19):6661–6668
Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB (2005) ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24(41):6314–6322
Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V (2006) Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Can Res 66(10):5287–5294
Clem BF, Clem AL, Yalcin A, Goswami U, Arumugam S, Telang S et al (2011) A novel small molecule antagonist of choline kinase-alpha that simultaneously suppresses MAPK and PI3K/AKT signaling. Oncogene 30(30):3370–3380
Flavin R, Peluso S, Nguyen PL, Loda M (2010) Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol 6(4):551–562
Mulvihill MM, Nomura DK (2013) Therapeutic potential of monoacylglycerol lipase inhibitors. Life Sci 92(8–9):492–497
Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GV, Oyajobi BO (2014) PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia 28(10):2080–2089
Kim MK, Yang S, Lee KH, Um JH, Liu M, Kang H et al (2011) Promyelocytic leukemia inhibits adipogenesis, and loss of promyelocytic leukemia results in fat accumulation in mice. Am J Physiol Endocrinol Metab 301(6):E1130–E1142
Yasugi E, Horiuchi A, Uemura I, Okuma E, Nakatsu M, Saeki K et al (2006) Peroxisome proliferator-activated receptor gamma ligands stimulate myeloid differentiation and lipogenensis in human leukemia NB4 cells. Dev Growth Differ 48(3):177–188
Le Y, Fraineau S, Chandran P, Sabloff M, Brand M, Lavoie JR et al (2016) Adipogenic mesenchymal stromal cells from bone marrow and their hematopoietic supportive role: towards understanding the permissive marrow microenvironment in acute myeloid leukemia. Stem Cell Rev 12(2):235–244
Samudio I, Fiegl M, Andreeff M (2009) Mitochondrial uncoupling and the Warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Can Res 69(6):2163–2166
Ricciardi MR, Mirabilii S, Allegretti M, Licchetta R, Calarco A, Torrisi MR et al (2015) Targeting the leukemia cell metabolism by the CPT1a inhibition: functional preclinical effects in leukemias. Blood 126(16):1925–1929
Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M et al (2016) Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell 19(1):23–37
Wu Y, Hurren R, MacLean N, Gronda M, Jitkova Y, Sukhai MA et al (2015) Carnitine transporter CT2 (SLC22A16) is over-expressed in acute myeloid leukemia (AML) and target knockdown reduces growth and viability of AML cells. Apoptosis Int J Program Cell Death 20(8):1099–1108
Lee EA, Angka L, Rota SG, Hanlon T, Mitchell A, Hurren R et al (2015) Targeting mitochondria with avocatin B induces selective leukemia cell death. Can Res 75(12):2478–2488
Velez J, Pan R, Lee JT, Enciso L, Suarez M, Duque JE et al (2016) Biguanides sensitize leukemia cells to ABT-737-induced apoptosis by inhibiting mitochondrial electron transport. Oncotarget 7(32):51435–51449
Messmer D, Lorrain K, Stebbins K, Bravo Y, Stock N, Cabrera G et al (2015) A selective novel peroxisome proliferator-activated receptor (PPAR)-alpha antagonist induces apoptosis and inhibits proliferation of CLL cells in vitro and in vivo. Mol Med 21:410–419
Shinohara H, Kumazaki M, Minami Y, Ito Y, Sugito N, Kuranaga Y et al (2016) Perturbation of energy metabolism by fatty-acid derivative AIC-47 and imatinib in BCR-ABL-harboring leukemic cells. Cancer Lett 371(1):1–11
Jacque N, Ronchetti AM, Larrue C, Meunier G, Birsen R, Willems L et al (2015) Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with BCL-2 inhibition. Blood 126(11):1346–1356
Goto M, Miwa H, Shikami M, Tsunekawa-Imai N, Suganuma K, Mizuno S et al (2014) Importance of glutamine metabolism in leukemia cells by energy production through TCA cycle and by redox homeostasis. Cancer Investig 32(6):241–247
Emadi A, Jun SA, Tsukamoto T, Fathi AT, Minden MD, Dang CV (2014) Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp Hematol 42(4):247–251
Herranz D, Ambesi-Impiombato A, Sudderth J, Sanchez-Martin M, Belver L, Tosello V et al (2015) Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in T cell acute lymphoblastic leukemia. Nat Med 21(10):1182–1189
van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D et al (2016) ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35(24):3201–3208
Polet F, Martherus R, Corbet C, Pinto A, Feron O (2016) Inhibition of glucose metabolism prevents glycosylation of the glutamine transporter ASCT2 and promotes compensatory LAT1 upregulation in leukemia cells. Oncotarget 7(29):46371–46383
Sontakke P, Koczula KM, Jaques J, Wierenga AT, Brouwers-Vos AZ, Pruis M et al (2016) Hypoxia-like signatures induced by BCR-ABL potentially alter the glutamine uptake for maintaining oxidative phosphorylation. PLoS ONE 11(4):e0153226
Polet F, Corbet C, Pinto A, Rubio LI, Martherus R, Bol V et al (2016) Reducing the serine availability complements the inhibition of the glutamine metabolism to block leukemia cell growth. Oncotarget 7(2):1765–1776
Alachkar H, Fulton N, Sanford B, Malnassy G, Mutonga M, Larson RA et al (2017) Expression and polymorphism (rs4880) of mitochondrial superoxide dismutase (SOD2) and asparaginase induced hepatotoxicity in adult patients with acute lymphoblastic leukemia. Pharmacogenomics J 17(3):274–279
Parmentier JH, Maggi M, Tarasco E, Scotti C, Avramis VI, Mittelman SD (2015) Glutaminase activity determines cytotoxicity of L-asparaginases on most leukemia cell lines. Leuk Res 39(7):757–762
Willems L, Jacque N, Jacquel A, Neveux N, Maciel TT, Lambert M et al (2013) Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 122(20):3521–3532
Chan WK, Lorenzi PL, Anishkin A, Purwaha P, Rogers DM, Sukharev S et al (2014) The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood 123(23):3596–3606
Chien WW, Le Beux C, Rachinel N, Julien M, Lacroix CE, Allas S et al (2015) Differential mechanisms of asparaginase resistance in B-type acute lymphoblastic leukemia and malignant natural killer cell lines. Sci Rep 5:8068
Lynn RC, Poussin M, Kalota A, Feng Y, Low PS, Dimitrov DS et al (2015) Targeting of folate receptor beta on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood 125(22):3466–3476
Uchiyama H, Sowa Y, Wakada M, Yogosawa M, Nakanishi R, Horinaka M et al (2010) Cyclin-dependent kinase inhibitor SU9516 enhances sensitivity to methotrexate in human T-cell leukemia Jurkat cells. Cancer Sci 101(3):728–734
Dulucq S, St-Onge G, Gagne V, Ansari M, Sinnett D, Labuda D et al (2008) DNA variants in the dihydrofolate reductase gene and outcome in childhood ALL. Blood 111(7):3692–3700
Teachey DT, Sheen C, Hall J, Ryan T, Brown VI, Fish J et al (2008) mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood 112(5):2020–2023
Wojtuszkiewicz A, Peters GJ, van Woerden NL, Dubbelman B, Escherich G, Schmiegelow K et al (2015) Methotrexate resistance in relation to treatment outcome in childhood acute lymphoblastic leukemia. J Hematol Oncol 8:61
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M et al (2010) Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA 107(19):8788–8793
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48(2):158–167
Zhou J, Bi C, Cheong LL, Mahara S, Liu SC, Tay KG et al (2011) The histone methyltransferase inhibitor, DZNep, up-regulates TXNIP, increases ROS production, and targets leukemia cells in AML. Blood 118(10):2830–2839
Doshi KA, Trotta R, Natarajan K, Rassool FV, Tron AE, Huszar D et al (2016) Pim kinase inhibition sensitizes FLT3-ITD acute myeloid leukemia cells to topoisomerase 2 inhibitors through increased DNA damage and oxidative stress. Oncotarget 7(30):48280–48295
Liu J, Masurekar A, Johnson S, Chakraborty S, Griffiths J, Smith D et al (2015) Stromal cell-mediated mitochondrial redox adaptation regulates drug resistance in childhood acute lymphoblastic leukemia. Oncotarget 6(40):43048–43064
Singh AK, Awasthi D, Dubey M, Nagarkoti S, Kumar A, Chandra T et al (2016) High oxidative stress adversely affects NFkappaB mediated induction of inducible nitric oxide synthase in human neutrophils: implications in chronic myeloid leukemia. Nitric Oxide 58:28–41
Acknowledgements
This study was supported by grants from Project of Shanghai Municipal Health Bureau (Surface Program, SHXH201402).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. BL designed the manuscript and TL and XP wrote the review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All authors are compliant with ethical standards.
Consent for publication
All authors approve the manuscript for publication.
Availability of data and materials
Data and materials related to this work are available upon request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, T., Peng, XC. & Li, B. The Metabolic Profiles in Hematological Malignancies. Indian J Hematol Blood Transfus 35, 625–634 (2019). https://doi.org/10.1007/s12288-019-01107-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-019-01107-8