Abstract
Background
Ovarian function suppression (OFS) plus other endocrine treatment was recommended to hormone receptor (HR)-positive breast cancer by some guidelines recently. We performed this study to validate the survival benefits of OFS plus aromatase inhibitors (AI) or selective estrogen receptor modulators (SERM) in the real world.
Methods
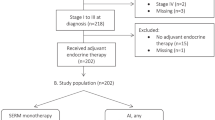
All premenopausal, HR-positive breast cancer patients diagnosed between 1996 and 2017 were identified. Eligible patients were classified into three groups according to their adjuvant endocrine treatment, including OFS plus AI, OFS plus SERM and SERM alone. The primary outcome is invasive disease-free survival (iDFS), whereas the secondary outcome is overall survival (OS). Cox proportional hazards models and propensity score adjusted models were used to compare the survival benefits in three groups.
Results
We included 2838 patients, of which 246 received OFS plus AI, 175 received OFS plus SERM, and 2417 received SERM alone. Compared with SERM alone, OFS plus AI was associated with an improved iDFS (HR 0.59, 95% CI 0.36–0.96) and OS (HR 0.26, 95% CI 0.08–0.85). OFS plus SERM, however, was not significantly associated with extended iDFS or OS. Among patients older than 40 years old, OFS plus AI was more effective than OFS plus SERM (HR 0.38, 95% CI 0.17–0.88) or SERM alone (HR 0.44, 95% CI 0.23–0.84) in terms of iDFS.
Conclusions
Our findings suggest that OFS plus AI treatment may extend both iDFS and OS among premenopausal patients with hormone receptor-positive breast cancer in the real world.



Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Singh L, Wilson AJ, Baum M, Whimster WF, Birch IH, Jackson IM, Lowrey C, Palmer MK. The relationship between histological grade, oestrogen receptor status, events and survival at 8 years in the NATO (‘Nolvadex’) trial. Br J Cancer. 1988;57(6):612–4.
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–16.
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–41.
(EBCTCG) EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717.
Goel S, Sharma R, Hamilton A, Beith J. LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women. Cochrane Database Syst Rev. 2009;4:Cd004562.
Griggs JJ, Somerfield MR, Anderson H, Henry NL, Hudis CA, Khatcheressian JL, Partridge AH, Prestrud AA, Davidson NE. American Society of Clinical Oncology endorsement of the cancer care Ontario practice guideline on adjuvant ovarian ablation in the treatment of premenopausal women with early-stage invasive breast cancer. J Clin Oncol. 2011;29(29):3939–42.
Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA, Burstein HJ, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–46.
Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, Gomez HL, Tondini C, Burstein HJ, Perez EA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–18.
Regan MM, Francis PA, Pagani O, Fleming GF, Walley BA, Viale G, Colleoni M, Lang I, Gomez HL, Tondini C, et al. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer: TEXT and SOFT trials. J Clin Oncol. 2016;34(19):2221–31.
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ. Tailoring therapies–improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26(8):1533–46.
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Solky AJ, Stearns V, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol. 2016;34(14):1689–701.
Bernhard J, Luo W, Ribi K, Colleoni M, Burstein HJ, Tondini C, Pinotti G, Spazzapan S, Ruhstaller T, Puglisi F, et al. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16(7):848–58.
Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360(7):679–91.
Chlebowski RT, Pan K, Col NF. Ovarian suppression in combination endocrine adjuvant therapy in premenopausal women with early breast cancer. Breast Cancer Res Treat. 2017;161(2):185–90.
Silverman SL. From randomized controlled trials to observational studies. Am J Med. 2009;122(2):114–20.
Roche N, Reddel H, Martin R, Brusselle G, Papi A, Thomas M, Postma D, Thomas V, Rand C, Chisholm A, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc. 2014;11(Suppl 2):99–104.
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017;28(8):1700–1712.
Peng Z, Wei J, Lu X, Zheng H, Zhong X, Gao W, Chen Y, Jing J. Treatment and survival patterns of Chinese patients diagnosed with breast cancer between 2005 and 2009 in Southwest China: an observational, population-based cohort study. Medicine. 2016;95(25):e3865.
Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH, et al. NCCN guidelines insights breast cancer, version 1.2016. J Natl Compr Cancer Netw JNCCN. 2015;13(12):1475–85.
Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, A’Hern R, Bliss J, Bogaerts J, Bonnefoi H, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials)dagger. Ann Oncol. 2015;26(5):873–9.
Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516–24.
Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. Jama. 2007;297(3):278–85.
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18(7):1133–44.
Xue C, Peng R, Cao Y, Wang S, Shi Y, An X, Xu F, Yuan Z. Ovarian function, not age, predicts the benefit from ovarian suppression or ablation for premenopausal women with breast cancer. PLoS One. 2016;11(2):e0148849.
Bosco JLF, Silliman RA, Thwin SS, Geiger AM, Buist DSM, Prout MN, Yood MU, Haque R, Wei F, Lash TL. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63(1):64–74.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81202099).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This study was approved by Biomedical Research Ethics Committee (approval number: 2012130), West China Hospital, Sichuan University and performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki and all subsequent revisions.
Informed consent
The informed consents were obtained from all participants. Permissions were, therefore, obtained to collect and store their related information, and used for medical research. All patients’ anonymity and privacy are carefully protected.
Conflict of interest
The authors declare that they have no potential conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hu, K., He, P., Peng, Q. et al. OFS plus AI or SERM vs. SERM alone in premenopausal women with hormone receptor-positive breast cancer: a prospective cohort study using the real-world database. Breast Cancer 26, 339–348 (2019). https://doi.org/10.1007/s12282-018-0929-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0929-6