Abstract
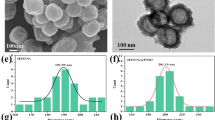
Multifunctional core–shell nanostructures formed by integration of distinct components have received wide attention as promising biological platforms in recent years. In this work, crystalline zeolitic imidazolate framework-8 (ZIF-8), a typical metal-organic framework (MOF), is coated onto single gold nanorod(AuNR) core for successful realization of synergistic photothermal and chemotherapy triggered by near-infrared (NIR) light. Impressively, high doxorubicin hydrochloride (DOX) loading capacity followed by pH and NIR light dual stimuli-responsive DOX release can be easily implemented through formation and breakage of coordination bonds in the system. Moreover, under NIR laser irradiation at 808 nm, these novel AuNR@MOF core–shell nanostructures exhibit effective synergistic chemo-photothermal therapy both in vitro and in vivo, confirmed by cell treatment and tumor ablation via intravenous injection.

Similar content being viewed by others
References
Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171.
Zhang, F. Y.; Shan, L.; Liu, Y. Y.; Neville, D.; Woo, J. H.; Chen, Y.; Korotcov, A.; Lin, S.; Huang, S.; Sridhar, R. et al. An anti-PSMA bivalent immunotoxin exhibits specificity and efficacy for prostate cancer imaging and therapy. Adv. Healthcare Mater. 2013, 2, 736–744.
Chen, G. Y.; Roy, I.; Yang, C. H.; Prasad, P. N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885.
Probst, C. E.; Zrazhevskiy, P.; Bagalkot, V.; Gao, X. H. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv. Drug Deliv. Rev. 2013, 65, 703–718.
Lim, E. K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y. M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394.
Liang, C.; Xu, L. G.; Song, G. S.; Liu, Z. Emerging nanomedicine approaches fighting tumor metastasis: Animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem. Soc. Rev. 2016, 45, 6250–6269.
Elsabahy, M.; Wooley, K. L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561.
Kemp, J. A.; Shim, M. S.; Heo, C. Y.; Kwon, Y. J. "Combo" nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18.
Cheng, L.; Wang, C.; Feng, L. Z.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939.
Elsabahy, M.; Heo, G. S.; Lim, S. M.; Sun, G. R.; Wooley, K. L. Polymeric nanostructures for imaging and therapy. Chem. Rev. 2015, 115, 10967–11011.
Yang, X.; Yang, M. X.; Pang, B.; Vara, M.; Xia, Y. N. Gold nanomaterials at work in biomedicine. Chem. Rev. 2015, 115, 10410–10488.
Moon, G. D.; Choi, S. W.; Cai, X.; Li, W. Y.; Cho, E. C.; Jeong, U.; Wang, L. V.; Xia, Y. N. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J. Am. Chem. Soc. 2011, 133, 4762–4765.
Liu, Y. J.; He, J.; Yang, K. K.; Yi, C. L.; Liu, Y.; Nie, L. M.; Khashab, N. M.; Chen, X. Y.; Nie, Z. H. Folding up of gold nanoparticles strings into plasmonic vesicles for enhanced photoacoustic imaging. Angew. Chem., Int. Ed. 2015, 54, 15809–15812.
Cui, T.; Liang, J. J.; Chen, H.; Geng, D. D.; Jiao, L.; Yang, J. Y.; Qian, H.; Zhang, C.; Ding, Y. Performance of doxorubicin-conjugated gold nanoparticles: Regulation of drug location. ACS Appl. Mater. Interfaces 2017, 9, 8569–8580.
Chen, H. J.; Shao, L.; Li, Q.; Wang, J. F. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724.
Lucky, S. S.; Soo, K. C.; Zhang, Y. Nanoparticles in Photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042.
You, J.; Shao, R. P.; Wei, X.; Gupta, S.; Li, C. Near-infrared light triggers release of paclitaxel from biodegradable microspheres: Photothermal effect and enhanced antitumor activity. Small 2010, 6, 1022–1031.
Li, W.; Zhang, X. J.; Zhou, M. J.; Tian, B. S.; Yu, C. Y.; Jie, J. S.; Hao, X. J.; Zhang, X. H. Functional core/shell drug nanoparticles for highly effective synergistic cancer therapy. Adv. Healthcare Mater. 2014, 3, 1475–1485.
Wu, G. H.; Mikhailovsky, A.; Khant, H. A.; Fu, C.; Chiu, W.; Zasadzinski, J. A. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J. Am. Chem. Soc. 2008, 130, 8175–8177.
Yavuz, M. S.; Cheng, Y. Y.; Chen, J. Y.; Cobley, C. M.; Zhang, Q.; Rycenga, M.; Xie, J. W.; Kim, C. H.; Song, K. H.; Schwartz, A. G. et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009, 8, 935–939.
Zhang, Z. J.; Wang, L. M.; Wang, J.; Jiang, X. M.; Li, X. H.; Hu, Z. J.; Ji, Y. L.; Wu, X. C.; Chen, C. Y. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv. Mater. 2012, 24, 1418–1423.
Croissant, J.; Zink, J. I. Nanovalve-controlled cargo release activated by plasmonic heating. J. Am. Chem. Soc. 2012, 134, 7628–7631.
Wang, S. Z.; McGuik, C. M.; Ross, M. B.; Wang, S. Y.; Chen, P. C.; Xing, H.; Liu, Y.; Mirkin, C. A. General and direct method for preparing oligonucleotide-functionalized metal-organic framework nanoparticles. J. Am. Chem. Soc. 2017, 139, 9827–9830.
Horcajada, P.; Gref, R.; Baati, T.; Allan, P. K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R. E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268.
He, C. B.; Liu, D. M.; Lin, W. B. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordination bonds: Nanoscale metal-organic frameworks and nanoscale coordination polymers. Chem. Rev. 2015, 115, 11079–11108.
Wang, H. S.; Li, J.; Li, J. Y.; Wang, K.; Ding, Y.; Xia, X. H. Lanthanide-based metal-organic framework nanosheets with unique fluorescence quenching properties for two-color intracellular adenosine imaging in living cells. NPG Asia Mater. 2017, 9, e354.
Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N. A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780.
Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J. F.; Heurtaux, D.; Clayette, P.; Kreuz, C. et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178.
Cai, G. R.; Jiang, H. L. A modulator-induced defect-formation strategy to hierarchically porous metal-organic frameworks with high stability. Angew. Chem., Int. Ed. 2017, 56, 563–567.
An, J.; Geib, S. J.; Rosi, N. L. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J. Am. Chem. Soc. 2009, 131, 8376–8377.
Wang, W. Q.; Wang, L.; Li, Z. S.; Xie, Z. G. BODIPY-con-taining nanoscale metal-organic frameworks for photodynamic therapy. Chem. Commun. 2016, 52, 5402–5405.
Yang, Y. Y.; Hu, Q.; Zhang, Q.; Jiang, K.; Lin, W. X.; Yang, Y.; Cui; Y. J.; Qian, G. D. A large capacity cationic metal-organic framework nanocarrier for physiological pH responsive drug delivery. Mol. Pharmaceutics 2016, 13, 2782–2786.
Zheng, H. Q.; Zhang, Y. N.; Liu, L. F.; Wan, W.; Guo, P.; Nyström, A. M.; Zou, X. D. One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016, 138, 962–968.
Wang, W. Q.; Wang, L.; Li, Y.; Liu, S.; Xie, Z. G.; Jing, X. B. Nanoscale polymer metal-organic framework hybrids for effective photothermal therapy of colon cancer. Adv. Mater. 2016, 28, 9320–9325.
Zheng, X. H.; Wang, L.; Pei, Q.; He, S. S.; Liu, S.; Xie, Z. G. Metal-organic framework@porous organic polymer nanocomposite for photodynamic therapy. Chem. Mater. 2017, 29, 2374–2381.
Lu, G.; Li, S. Z.; Guo, Z.; Farha, O. K.; Hauser, B. G.; Qi, X. Y.; Wang, Y.; Wang, X.; Han, S. Y.; Liu, X. G. et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316.
Sun, C. Y.; Qin, C.; Wang, X. L.; Yang, G. S.; Shao, K. Z.; Lan, Y. Q.; Su, Z. M.; Huang, P.; Wang, C. G.; Wang, E. B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909.
Ren, H.; Zhang, L. Y.; An, J. P.; Wang, T. T.; Li, L.; Si, X. Y.; He, L.; Wu, X. T.; Wang, C. G.; Su, Z. M. Polyacrylic acid@zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release. Chem. Commun. 2014, 50, 1000–1002.
Wang, Z. F.; Tang, X. J.; Wang X. X.; Yang, D. D.; Yang, C.; Lou, Y. B.; Chen, J. X.; He, N. Y. Near-infrared light-induced dissociation of zeolitic imidazole framework-8 (ZIF-8) with encapsulated CuS nanoparticles and their application as a therapeutic nanoplatform. Chem. Commun. 2016, 52, 12210–12213.
Tian, Z. F.; Yao, X. X.; Ma, K. X.; Niu, X. X.; Grothe, J. L.; Xu, Q. N.; Liu, L. S.; Kaskel, S.; Zhu, Y. F. Metal-organic framework/graphene quantum dot nanoparticles used for synergistic chemo- and photothermal therapy. ACS Omega 2017, 2, 1249–1258.
Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282.
Cobley, C. M.; Chen, J. Y.; Cho, E. C.; Wang, L. V.; Xia, Y. N. Gold nanostructures: A class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56.
Nikoobakht, B.; El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962.
He, L. C.; Liu, Y.; Liu, J. Z.; Xiong, Y. S.; Zheng, J. Z.; Liu, Y. L.; Tang, Z. Y. Core-shell noble-metal@metal-organic-frame-work nanoparticles with highly selective sensing property. Angew. Chem., Int. Ed. 2013, 52, 3741–3745.
Liu, X.; He, L. C.; Zheng, J. Z.; Guo, J.; Bi, F.; Ma, X.; Zhao, K.; Liu, Y. L.; Song, R.; Tang, Z. Y. Solar-light-driven renewable butanol separation by core-shell Ag@ZIF-8 nanowires. Adv. Mater. 2015, 27, 3273–3277.
Li, Y. T.; Tang, J. L.; He, L. C.; Liu, Y.; Liu, Y. L.; Chen, C. Y.; Tang, Z. Y. Core-shell upconversion nanoparticle@metal-organic framework nanoprobes for luminescent/magnetic dual-mode targeted imaging. Adv. Mater. 2015, 27, 4075–4080.
Hayashi, H.; Côté, A. P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O. M. Zeolite a imidazolate frameworks. Nat. Mater. 2007, 6, 501–506.
Abraham, S. A.; Edwards, K.; Karlsson, G.; MacIntosh, S.; Mayer, L. D.; McKenzie, C.; Bally, M. B. Formation of transition metal-doxorubicin complexes inside liposomes. Biochim. Biophys. Acta, Biomembr. 2002, 1565, 41–54.
Barick, K. C.; Nigam S.; Bahadur, D. Nanoscale assembly of mesoporous ZnO: A potential drug carrier. J. Mater. Chem. 2010, 20, 6446–6452.
Vasconcelos, I. B.; da Silva, T. G.; Militão, G. C. G.; Soares, T. A.; Rodrigues, N. M.; Rodrigues, M. O.; da Costa, N. B., Jr.; Freire, R. O.; Junior, S. A. Cytotoxicity and slow release of the anti-cancer drug doxorubicin from ZIF-8. RSC Adv. 2012, 2, 9437–9442.
Yang, J. P.; Shen, D. K.; Zhou, L.; Li, W.; Li, X. M.; Yao, C.; Wang, R.; El-Toni, A. M.; Zhang, F.; Zhao, D. Y. Spatially confine fabrication of core-shell gold nanocages@-mesoporous silica for near-infrared controlled photothermal drug release. Chem. Mater. 2013, 25, 3030–3037.
Zhang, L. Y.; Chen, Y. Y.; Li, Z. L.; Li, L.; Saint-Cricq, P.; Li, C. X.; Lin, J.; Wang, C. G.; Su, Z. M.; Zink, J. I. Tailored synthesis of octopus-type janus nanoparticles for synergistic actively-tar-geted and chemo-photothermal therapy. Angew. Chem., Int. Ed. 2016, 55, 2118–2121.
Chen, R.; Zhang, J. F.; Wang, Y.; Chen, X. F.; Zapien, J. A.; Lee, C. S. Graphitic carbon nitride nanosheet@metal-organic framework core-shell nanoparticles for photo-chemo combination therapy. Nanoscale 2015, 7, 17299–17305.
Chen, X. J.; Zhang, M. J.; Li, S. N.; Li, L.; Zhang, L. Y.; Wang, T. T.; Yu, M.; Mou, Z. C.; Wang, C. G. Facile synthesis of polypyrrole@metal-organic framework core-shell nanocomposites for dual-mode imaging and synergistic chemo-photothermal therapy of cancer cells. J. Mater. Chem. B 2017, 5, 1772–1778.
Ke, F.; Yuan, Y. P.; Qiu, L. G.; Shen, Y. H.; Xie, A. J.; Zhu, J. F.; Tian, X. Y.; Zhang, L. D. Facile fabrication of magnetic metal-organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21, 3843–3848.
Zhang, F. M.; Dong, H.; Zhang, X.; Sun, X. J.; Liu, M.; Yang, D. D.; Liu, X.; Wei, J. Z. Postsynthetic modification of ZIF-90 for potential targeted codelivery of two anticancer drugs. ACS Appl. Mater. Interfaces 2017, 9, 27332–27337.
Zheng, H. Q.; Xing, L.; Cao, Y. Y.; Che, S. A. Coordination bonding based pH-responsive drug delivery systems. Coord. Chem. Rev. 2013, 257, 1933–1944.
He, M. N.; Zhou, J. J.; Chen, J.; Zheng, F. C.; Wang, D. D.; Shi, R. H.; Guo, Z.; Wang, H. B.; Chen, Q. W. Fe3O4@carbon@zeolitic imidazolate framework-8 nanoparticles as multifunctional pH-responsive drug delivery vehicles for tumor therapy in vivo. J. Mater. Chem. B 2015, 3, 9033–9042.
Park, K. S.; Ni, Z.; Côté, A. P.; Choi, J. Y.; Huang, R. D.; Uribe-Romo, F. J.; Chae, H. K.; Keeffe, M. O.; Yaghi, O. M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191.
Fang, W. J.; Yang, J.; Gong, J. W.; Zheng, N. F. Photo- and pH-triggered release of anticancer drugs from mesoporous silica-coated Pd@Ag nanoparticles. Adv. Funct. Mater. 2012, 22, 842–848.
Zhang, X. L.; Jiang, J. W. Thermal conductivity of zeolitic imidazolate framework-8: A molecular simulation study. J. Phys. Chem. C 2013, 117, 18441–18447.
Sassaroli, E.; Li, K. C. P.; O’Neill, B. E. Numerical investigation of heating of a gold nanoparticle and the surrounding microenvironment by nanosecond laser pulses for nanomedicine applications. Phys. Med. Biol. 2009, 54, 5541–5560.
Baffou, G.; Girard, C.; Quidant, R. Mapping heat origin in plasmonic structures. Phys. Rev. Lett. 2010, 104, 136805.
Baffou, G.; Quidant, R.; de Abajo, F. J. G. Nanoscale control of optical heating in complex plasmonic systems. ACS Nano 2010, 4, 709–716.
Lv, R. C.; Yang, P. P.; He, F.; Gai, S. L.; Li, C. X.; Dai, Y. L.; Yang, G. X.; Lin, J. A yolk-like multifunctional platform for multimodal imaging and synergistic therapy triggered by a single near-infrared light. ACS Nano 2015, 9, 1630–1647.
Wang, Y.; Wang, K. Y.; Zhang, R.; Liu, X. G.; Yan, X. Y.; Wang, J. X.; Wagner, E.; Huang, R. Q. Synthesis of core–shell graphitic carbon@silica nanospheres with dual-ordered mesopores for cancer-targeted photothermochemotherapy. ACS Nano 2014, 8, 7870–7879.
Kirui, D. K.; Celia, C.; Molinaro, R.; Bansal, S. S.; Cosco, D.; Fresta, M.; Shen, H. F.; Ferrari, M. Mild hyperthermia enhances transport of liposomal gemcitabine and improves in vivo therapeutic response. Adv. Healthcare Mater. 2015, 4, 1092–1103.
Kirui, D. K.; Koay, E. J.; Guo, X. J.; Cristini, V.; Shen, H. F.; Ferrari, M. Tumor vascular permeabilization using localized mild hyperthermia to improve macromolecule transport. Nanomedicine 2014, 10, 1487–1496.
Chae, W. J.; Gibson, T. F.; Zelterman, D.; Hao, L. M.; Henegariu, O.; Bothwell, A. L. M. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 5540–5544.
Acknowledgements
This work was supported financially by National Basic Research Program of China (Nos. 2014CB93-1801 and 2016YFA0200700, Z. Y. T.), National Natural Science Foundation for Distinguished Youth Scholars of China (No. 11425520, C. Y. C.), National Natural Science Foundation of China (Nos. 21721002, 21475029, and 91427302, Z. Y. T.; 21722301 and 21371038, Y. L. L.), Frontier Science Key Project of the Chinese Academy of Sciences (No. QYZDJ-SSW-SLH038, Z. Y. T.), CAS-CSIRO Cooperative Research Program (No. GJHZ1503, Z. Y. T.), “Strategic Priority Research Program” of Chinese Academy of Sciences (No. XDA09040100, Z. Y. T.; XDA09030308, Y. L. L.), Youth Innovation Promotion Association of Chinese Academy of Sciences (Y. L. L.), and K. C. Wong Education Foundation (Z. Y. T.).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2017_1874_MOESM1_ESM.pdf
Coordination-responsive drug release inside gold nanorod@metal-organic framework core–shell nanostructures for near-infrared-induced synergistic chemo-photothermal therapy
Rights and permissions
About this article
Cite this article
Li, Y., Jin, J., Wang, D. et al. Coordination-responsive drug release inside gold nanorod@metal-organic framework core–shell nanostructures for near-infrared-induced synergistic chemo-photothermal therapy. Nano Res. 11, 3294–3305 (2018). https://doi.org/10.1007/s12274-017-1874-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1874-y