Abstract
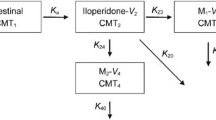
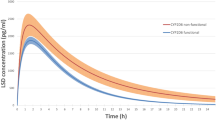
To investigate the effect of the variant CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites, 4-hydroxyatomoxetine (4-HAT) and N-desmethylatomoxetine (NAT), in healthy subjects, a single oral dose of atomoxetine was administered to 62 subjects with a CYP2D6*wt/*wt (*wt = *1 or *2, n = 22), CYP2D6*wt/*10 (n = 22) or CYP2D6*10/*10 (n = 18) genotype. Plasma samples were then collected for 24 h after atomoxetine administration. The concentrations of atomoxetine and its metabolites were assayed using LC–MS/MS. For atomoxetine, the Cmax, AUC0–∞, t1/2 and CL/F showed genotype-dependent differences. The CYP2D6*10/*10 and CYP2D6*wt/*10 groups showed 1.74- and 1.15-fold higher Cmax, 3.40- and 1.33-fold higher AUC0–∞, and 69.7 and 24.6 % lower CL/F, compared to those of the CYP2D6*wt/*wt group, respectively. The Cmax and t1/2 for 4-HAT were lower and longer in the CYP2D6*10/*10 group than those in the CYP2D6*wt/*wt group, but the AUC0–∞ was not different between these groups. The Cmax, AUC0–∞ and t1/2 for NAT were profoundly greater in the CYP2D6*10/*10 group than they were in the CYP2D6*wt/*wt group. The concentration of active moieties of atomoxetine (atomoxetine + 4-HAT) in the CYP2D6*10/*10 group was 3.32-fold higher than that in the CYP2D6*wt/*wt group. The mean exposure to active moieties of atomoxetine was markedly higher in subjects with the CYP2D6*10/*10 genotype compared to that in those with the CYP2D6*wt/*wt genotype. The higher systemic exposure of the active atomoxetine moieties in CYP2D6*10/*10 individuals may increase the risk of concentration-related adverse events of atomoxetine, although this has not yet been clinically confirmed.


Similar content being viewed by others
References
Barker, M.J., J.G. Benitez, S. Ternullo, and G.A. Juhl. 2004. Acute oxcarbazepine and atomoxetine overdose with quetiapine. Veterinary and Human Toxicology 46: 130–132.
Choi, C.I., J.W. Bae, H.I. Lee, C.G. Jang, U.D. Sohn, and S.Y. Lee. 2012a. Determination of atomoxetine metabolites in human plasma by liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study. Journal of Chromatography B 885: 103–108.
Choi, C.I., C.G. Jang, J.W. Bae, and S.Y. Lee. 2012b. Validation of an analytical LC-MS/MS method in human plasma for the pharmacokinetic study of atomoxetine. Journal of Analytical Chemistry 68: 986–991.
Corman, S.L., B.A. Fedutes, and C.M. Culley. 2004. Atomoxetine: the first nonstimulant for the management of attention-deficit/hyperactivity disorder. American Journal of Health System Pharmacy 61: 2391–2399.
Cui, Y.M., C.H. Teng, A.X. Pan, E. Yuen, K.P. Yeo, Y. Zhou, X. Zhao, A.J. Long, M.E. Bangs, and S.D. Wise. 2007. Atomoxetine pharmacokinetics in healthy Chinese subjects and effect of the CYP2D6*10 allele. British Journal of Clinical Pharmacology 64: 445–449.
Dupont, W.D., and W.D. Plumer. 1998. Power and sample size calculations for studies involving linear regression. Cotrol Clinical Trials 19: 589–601.
Gehlert, D.R., S.L. Gackenheimer, and D.W. Robertson. 1993. Localization of rat brain binding sites for [3H]tomoxetine, an enantiomerically pure ligand for norepinephrine reuptake sites. Neuroscience Letters 157: 203–206.
Ingelman-Sundberg, M., S.C. Sim, A. Gomez, and C. Rodriguez-Antona. 2007. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacology & Therapeutics 116: 496–526.
Ingelman-Sundberg, M. 2005. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics Journal 5: 6–13.
Ji, L., S. Pan, J. Marti-Jaun, E. Hänseler, K. Rentsch, and M. Hersberger. 2002. Single-step assays to analyze CYP2D6 gene polymorphisms in Asians: allele frequencies and a novel *14B allele in mainland Chinese. Clinical Chemistry 48: 983–988.
Jin, S.K., H.J. Chung, M.W. Chung, J.I. Kim, J.H. Kang, S.W. Woo, S. Bang, S.H. Lee, H.J. Lee, and J. Roh. 2008. Influence of CYP2D6*10 on the pharmacokinetics of metoprolol in healthy Korean volunteers. Journal of Clinical Pharmacy and Therapeutics 33: 567–573.
Johansson, I., E. Lundqvist, M.L. Dahl, and M. Ingelman-Sundberg. 1996. PCR-based genotyping for duplicated and deleted CYP2D6 genes. Pharmacogenetics 6: 351–355.
Kasi, P.M., R. Mounzer, and G.H. Gleeson. 2011. Cardiovascular side effects of atomoxetine and its interactions with inhibitors of the cytochrome p450 system. Case Report in Medicine 2011: 952584.
Lee, S.J., S.S. Lee, H.J. Jung, H.S. Kim, S.J. Park, C.W. Yeo, and J.G. Shin. 2009. Discovery of novel functional variants and extensive evaluation of CYP2D6 genetic polymorphisms in Koreans. Drug Metabolism and Disposition 37: 1464–1470.
Li, Q., R. Wang, Y. Guo, S. Wen, L. Xu, and S. Wang. 2010. Relationship of CYP2D6 genetic polymorphisms and the pharmacokinetics of tramadol in Chinese volunteers. Journal of Clinical Pharmacy and Therapeutics 35: 239–247.
Lundqvist, E., I. Johansson, and M. Ingelman-Sundberg. 1999. Genetic mechanisms for duplication and multiduplication of the human CYP2D6 gene and methods for detection of duplicated CYP2D6 genes. Gene 226: 327–338.
Marez, D., M. Legrand, N. Sabbagh, J.M. Lo Guidice, C. Spire, J.J. Lafitte, U.A. Meyer, and F. Broly. 1997. Polymorphism of the cytochrome P450 CYP2D6 gene in a European population: characterization of 48 mutations and 53 alleles, their frequencies and evolution. Pharmacogenetics 7: 193–202.
Matsui, A., J. Azuma, J.W. Witcher, A.J. Long, J.M. Sauer, B.P. Smith, K.A. DeSante, H.A. Read, M. Takahashi, and M. Nakano. 2012. Pharmacokinetics, Safety, and Tolerability of Atomoxetine and Effect of CYP2D6*10/*10 Genotype in Healthy Japanese Men. Journal of Clinical Pharmacology 52: 388–403.
Mendoza, R., Y.J. Wan, R.E. Poland, M. Smith, Y. Zheng, N. Berman, and K.M. Lin. 2001. CYP2D6 polymorphism in a Mexican American population. Clinical Pharmacology and Therapeutics 70: 552–560.
Michelson, D., H.A. Read, D.D. Ruff, J. Witcher, S. Zhang, and J. McCracken. 2007. CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry 46: 242–251.
Nishida, Y., T. Fukuda, I. Yamamoto, and J. Azuma. 2000. CYP2D6 genotypes in a Japanese population: low frequencies of CYP2D6 gene duplication but high frequency of CYP2D6*10. Pharmacogenetics 10: 567–570.
Paulzen, M., H.W. Clement, and G. Gründer. 2008. Enhancement of atomoxetine serum levels by co-administration of paroxetine. International Journal of Neuropsychopharmacology 11: 289–291.
Ring, B.J., J.S. Gillespie, J.A. Eckstein, and S.A. Wrighton. 2002. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metabolism and Disposition 30: 319–323.
Sauer, J.M., G.D. Ponsler, E.L. Mattiuz, A.J. Long, J.W. Witcher, H.R. Thomasson, and K.A. Desante. 2003. Disposition and metabolic fate of atomoxetine hydrochloride: the role of CYP2D6 in human disposition and metabolism. Drug Metabolism and Disposition 31: 98–107.
Sauer, J.M., B.J. Ring, and J.W. Witcher. 2005. Clinical pharmacokinetics of atomoxetine. Clinical Pharmacokinetics 44: 571–590.
Sawant, S., and S.R. Daviss. 2004. Seizures and prolonged QTc with atomoxetine overdose. American Journal of Psychiatry 161: 757.
Scherer, D., D. Hassel, R. Bloehs, E. Zitron, K. von Löwenstern, C. Seyler, D. Thomas, F. Konrad, H.F. Bürgers, G. Seemann, W. Rottbauer, H.A. Katus, C.A. Karle, and E.P. Scholz. 2009. Selective noradrenaline reuptake inhibitor atomoxetine directly blocks hERG currents. British Journal of Pharmacology 156: 226–236.
Simpson, D., and G.L. Plosker. 2004. Atomoxetine: A review of its use in adults with attention deficit hyperactivity disorder. Drugs 64: 205–222.
Steimer, W., K. Zöpf, S. von Amelunxen, H. Pfeiffer, J. Bachofer, J. Popp, B. Messner, W. Kissling, and S. Leucht. 2004. Allele-specific change of concentration and functional gene dose for the prediction of steady-state serum concentrations of amitriptyline and nortriptyline in CYP2C19 and CYP2D6 extensive and intermediate metabolizers. Clinical Chemistry 50: 1623–1633.
ter Laak, M.A., A.H. Temmink, A. Koeken, N.E. van’t Veer, P.R. van Hattum, and C.M. Cobbaert. 2010. Recognition of impaired atomoxetine metabolism because of low CYP2D6 activity. Pediatric Neurology 43: 159–162.
Wang, S.L., J.D. Huang, M.D. Lai, B.H. Liu, and M.L. Lai. 1993. Molecular basis of genetic variation in debrisoquin hydroxylation in Chinese subjects: polymorphism in RFLP and DNA sequence of CYP2D6. Clinical Pharmacology and Therapeutics 53: 410–418.
Wernicke, J.F., D. Faries, D. Girod, J. Brown, H. Gao, D. Kelsey, H. Quintana, R. Lipetz, D. Michelson, and J. Heiligenstein. 2003. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Safety 26: 729–740.
Zhou, Q., X.M. Yu, H.B. Lin, L. Wang, Q.Z. Yun, S.N. Hu, and D.M. Wang. 2009. Genetic polymorphism, linkage disequilibrium, haplotype structure and novel allele analysis of CYP2C19 and CYP2D6 in Han Chinese. Pharmacogenomics Journal 9: 380–394.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We would like to declare that there were no conflicts of interest in conducting this research.
Additional information
Ji-Yeong Byeon, Young-Hoon Kim, Han-Sung Na and Jong-Hwa Jang have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Byeon, JY., Kim, YH., Na, HS. et al. Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch. Pharm. Res. 38, 2083–2091 (2015). https://doi.org/10.1007/s12272-015-0646-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0646-z