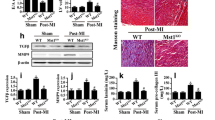
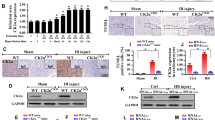
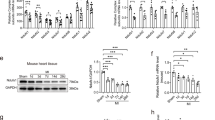
Abstract
Mitochondrial protein sequence similarity 3 gene family member A (FAM3A) plays important roles in the electron transfer chain, while its functions in the heart are still unknown. This study aims to explore the roles and mechanisms of FAM3A after myocardial infarction (MI). FAM3A-deficient (Fam3a−/−) mice were implemented with MI injury and showed lower survival rates at 4 weeks as well as decreased cardiac systolic function. Isolated cardiomyocytes of Fam3a−/− mice showed reduced basal, ATP-linked respiration and respiratory reserve compared to that of wild-type mice. Transmission electron microscopy studies showed Fam3a−/− mice had a larger size and elevated density of mitochondria. FAM3A deficiency also induced elevated mitochondrial Ca2+, higher opening level of mPTP, lower mitochondrial membrane potential and elevated apoptotic rates. Further analyses demonstrated that mitochondrial dynamics protein Opa1 contributed to the effects of FAM3A in cardiomyocytes. Our study discloses the important roles of mitochondrial protein FAM3A in the heart.
Graphical abstract







Similar content being viewed by others
Data Availability
Data supporting the findings of this study are available within the article and its supplementary materials.
References
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol. 2021;2021. https://doi.org/10.1093/eurjpc/zwab154
Yue R, Xia X, Jiang J, Yang D, Han Y, Chen X, Cai Y, Li L, Wang WE, Zeng C. Mitochondrial DNA oxidative damage contributes to cardiomyocyte ischemia/reperfusion-injury in rats: cardioprotective role of lycopene. J Cell Physiol. 2015;230(9):2128–41. https://doi.org/10.1002/jcp.24941.
Sun N, Finkel T. Cardiac mitochondria: a surprise about size. J Mol Cell Cardiol. 2015;82:213–5. https://doi.org/10.1016/j.yjmcc.2015.01.009.
Virmani R, Forman MB, Kolodgie FD. Myocardial reperfusion injury. Histopathological effects of perfluorochemical. Circulation. 1990;81(3 Suppl):Iv57-68
Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018;19(11):713–30. https://doi.org/10.1038/s41580-018-0052-8.
Zhu H, Toan S, Mui D, Zhou H. Mitochondrial quality surveillance as a therapeutic target in myocardial infarction. Acta Physiol (Oxf). 2021;231(3):e13590. https://doi.org/10.1111/apha.13590.
Zhou H, Ren J, Toan S, Mui D. Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res Rev. 2021;6:101250. https://doi.org/10.1016/j.arr.2020.101250.
Ramachandra CJA, Hernandez-Resendiz S, Crespo-Avilan GE, Lin YH, Hausenloy DJ. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine. 2020;57:102884. https://doi.org/10.1016/j.ebiom.2020.102884.
Anzell AR, Maizy R, Przyklenk K, Sanderson TH. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol. 2018;55(3):2547–64. https://doi.org/10.1007/s12035-017-0503-9.
Wang C, Chi Y, Li J, Miao Y, Li S, Su W, Jia S, Chen Z, Du S, Zhang X, Zhou Y, Wu W, Zhu M, Wang Z, Yang H, Xu G, Wang S, Yang J, Guan Y. FAM3A activates PI3K p110α/Akt signaling to ameliorate hepatic gluconeogenesis and lipogenesis. Hepatology. 2014;59(5):1779–90. https://doi.org/10.1002/hep.26945.
Yang W, Wang J, Chen Z, Chen J, Meng Y, Chen L, Chang Y, Geng B, Sun L, Dou L, Li J, Guan Y, Cui Q, Yang J. NFE2 induces miR-423-5p to promote gluconeogenesis and hyperglycemia by repressing the hepatic FAM3A-ATP-Akt pathway. Diabetes. 2017;66(7):1819–32. https://doi.org/10.2337/db16-1172.
Zhang X, Yang W, Wang J, Meng Y, Guan Y, Yang J. FAM3 gene family: a promising therapeutical target for NAFLD and type 2 diabetes. Metabolism. 2018;81:71–82. https://doi.org/10.1016/j.metabol.2017.12.001.
Song Q, Gou WL, Zhang R. FAM3A attenuates ER stress-induced mitochondrial dysfunction and apoptosis via CHOP-Wnt pathway. Neurochem Int. 2016;94:82–9. https://doi.org/10.1016/j.neuint.2016.02.010.
Chen Z, Wang J, Yang W, Chen J, Meng Y, Geng B, Cui Q, Yang J. FAM3A mediates PPARgamma's protection in liver ischemia-reperfusion injury by activating Akt survival pathway and repressing inflammation and oxidative stress. Oncotarget. 2017;8(30):49882-96. https://doi.org/10.18632/oncotarget.17805
Chen Z, Liu X, Luo Y, Wang J, Meng Y, Sun L, Chang Y, Cui Q, Yang J. Repurposing doxepin to ameliorate steatosis and hyperglycemia by activating FAM3A signaling pathway. Diabetes. 2020;69(6):1126–39. https://doi.org/10.2337/db19-1038.
Wang J, Zhang J, Lin X, Wang Y, Wu X, Yang F, Gao W, Zhang Y, Sun J, Jiang C, Xu M. DCA-TGR5 signaling activation alleviates inflammatory response and improves cardiac function in myocardial infarction. J Mol Cell Cardiol. 2021;151:3–14. https://doi.org/10.1016/j.yjmcc.2020.10.014.
Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279(1):H429–36. https://doi.org/10.1152/ajpheart.2000.279.1.H429.
Zhao YT, Guo YB, Gu L, Fan XX, Yang HQ, Chen Z, Zhou P, Yuan Q, Ji GJ, Wang SQ. Sensitized signalling between L-type Ca2+ channels and ryanodine receptors in the absence or inhibition of FKBP12.6 in cardiomyocytes. Cardiovasc Res. 2017;113(3):332–42. https://doi.org/10.1093/cvr/cvw247
Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007;2(2):287–95. https://doi.org/10.1038/nprot.2006.478.
Zhu M, Gao J, Lin XJ, Gong YY, Qi YC, Ma YL, Song YX, Tan W, Li FY, Ye M, Gong J, Cui QH, Huang ZH, Zhang YY, Wang XJ, Lan F, Wang SQ, Yuan G, Feng Y, Xu M. Novel roles of an intragenic G-quadruplex in controlling microRNA expression and cardiac function. Nucleic Acids Res. 2021;49(5):2522–36. https://doi.org/10.1093/nar/gkab055.
Lee H, Yoon Y. Mitochondrial membrane dynamics-functional positioning of OPA1. Antioxidants (Basel). 2018;7(12). https://doi.org/10.3390/antiox7120186
Zhu Y, Xu G, Patel A, McLaughlin MM, Silverman C, Knecht K, Sweitzer S, Li X, McDonnell P, Mirabile R, Zimmerman D, Boyce R, Tierney LA, Hu E, Livi GP, Wolf B, Abdel-Meguid SS, Rose GD, Aurora R, Hensley P, Briggs M, Young PR. Cloning, expression, and initial characterization of a novel cytokine-like gene family. Genomics. 2002;80(2):144–50. https://doi.org/10.1006/geno.2002.6816.
Xiang R, Chen J, Li S, Yan H, Meng Y, Cai J, Cui Q, Yang Y, Xu M, Geng B, Yang J. VSMC-Specific deletion of FAM3A attenuated Ang II-promoted hypertension and cardiovascular hypertrophy. Circ Res. 2020;126(12):1746–59. https://doi.org/10.1161/CIRCRESAHA.119.315558.
Xu W, Liang M, Zhang Y, Huang K, Wang C. Endothelial FAM3A positively regulates post-ischaemic angiogenesis. EBioMedicine. 2019;43:32–42. https://doi.org/10.1016/j.ebiom.2019.03.038.
Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17(12):773–89. https://doi.org/10.1038/s41569-020-0403-y.
Heusch G. Myocardial stunning and hibernation revisited. Nat Rev Cardiol. 2021;18(7):522–36. https://doi.org/10.1038/s41569-021-00506-7.
Verma DD, Hartner WC, Levchenko TS, Bernstein EA, Torchilin VP. ATP-loaded liposomes effectively protect the myocardium in rabbits with an acute experimental myocardial infarction. Pharm Res. 2005;22(12):2115–20. https://doi.org/10.1007/s11095-005-8354-x.
Hartner WC, Verma DD, Levchenko TS, Bernstein EA, Torchilin VP. ATP-loaded liposomes for treatment of myocardial ischemia. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(5):530–9. https://doi.org/10.1002/wnan.46.
Xin Y, Zhang X, Li J, Gao H, Li J, Li J, Hu W, Li H. New insights into the role of mitochondria quality control in ischemic heart disease. Front Cardiovasc Med. 2021;8:774619. https://doi.org/10.3389/fcvm.2021.774619.
Schipper DA, Palsma R, Marsh KM, O’Hare C, Dicken DS, Lick S, Kazui T, Johnson K, Smolenski RT, Duncker DJ, Khalpey Z. Chronic myocardial ischemia leads to loss of maximal oxygen consumption and complex I dysfunction. Ann Thorac Surg. 2017;104(4):1298–304. https://doi.org/10.1016/j.athoracsur.2017.03.004.
Stride N, Larsen S, Hey-Mogensen M, Hansen CN, Prats C, Steinbrüchel D, Køber L, Dela F. Impaired mitochondrial function in chronically ischemic human heart. Am J Physiol Heart Circ Physiol. 2013;304(11):H1407–14. https://doi.org/10.1152/ajpheart.00991.2012.
Dorn GW 2nd, Kitsis RN. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res. 2015;116(1):167–82. https://doi.org/10.1161/CIRCRESAHA.116.303554.
Davidson SM. FAM3A - A mitochondrial route to the stimulation of angiogenesis? EBioMedicine. 2019;43:3–4. https://doi.org/10.1016/j.ebiom.2019.04.033.
Pernas L, Scorrano L. Mito-Morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu Rev Physiol. 2016;78:505–31. https://doi.org/10.1146/annurev-physiol-021115-105011.
Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, Mateo P, Fortin D, le Huynh H, Nicolas V, Alavi MV, Brenner C, Ventura-Clapier R, Veksler V, Joubert F. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res. 2012;94(3):408–17. https://doi.org/10.1093/cvr/cvs117.
Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187(7):959–66. https://doi.org/10.1083/jcb.200906083.
Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187(7):1023–36. https://doi.org/10.1083/jcb.200906084.
McBride H, Soubannier V. Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr Biol. 2010;20(6):R274–6. https://doi.org/10.1016/j.cub.2010.02.011.
Wang M, Wang RY, Zhou JH, Xie XH, Sun GB, Sun XB. Calenduloside E Ameliorates myocardial ischemia-reperfusion injury through regulation of AMPK and mitochondrial OPA1. Oxid Med Cell Longev. 2020;2020:2415269. https://doi.org/10.1155/2020/2415269.
Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155(1):160–71. https://doi.org/10.1016/j.cell.2013.08.032.
Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabò R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L. The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab. 2015;21(6):834–44. https://doi.org/10.1016/j.cmet.2015.05.007.
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–33. https://doi.org/10.1152/ajpcell.00139.2004.
Kuznetsov AV, Javadov S, Margreiter R, Grimm M, Hagenbuchner J, Ausserlechner MJ. The role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidants (Basel). 2019;8(10). https://doi.org/10.3390/antiox8100454
Acknowledgements
We thank professor Xin Pan (State Key Laboratory of Toxicology and Medical Countermeasures, Institute of Pharmacology and Toxicology, National Center of Biomedical Analysis, Beijing, China) for constructive criticism in the project design.
Funding
This work was supported by: Research Unit of Medical Science Research Management/Basic and Clinical Research of Metabolic Cardiovascular Diseases, Chinese Academy of Medical Sciences (2021RU003), National Natural Science Foundation of China (Grant No. 82270343, No. 81900315, No. U20A20345) and National Key Research and Development Program of China (No.2020YFA0803800, No. 2020YFA0803803).
Author information
Authors and Affiliations
Contributions
TX, JXW, and XXL performed the experiments and analyzed the data. TX, JXW, and XXL wrote the manuscript. RX, HHL and SQW provided experimental advice and some experimental supplies. JCY and MX designed the experiments. All authors contributed to the editing of the review and approved the final version of the manuscript for submission.
Corresponding authors
Ethics declarations
Conflict of Interest
None declared.
Additional information
Associate Editor Yihua Bei oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12265_2023_10382_MOESM1_ESM.pptx
Supplementary file1 (PPTX 65865 KB) Figure S1. (A) Serum level of cardiac troponin T 6 hours post-MI (n=6). (B) Lung edema measured by wet-dry weight of sham and 28-day post-MI (n=6). (C) Heart to tibia weight ratio at 28-day post-MI (n=6). Figure S2. (A-D) representative pictures and (E) statistics analysis of protein level of Mfn1, Mfn2, DRP1, and Fis1 from cardiac infarct border tissues at 7-day post-MI or sham heart tissue. ns, non-significant. All values are presented as mean ± SEM. Data were analyzed by two-way ANOVA, followed by subsequent post hoc multiple comparison.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, T., Wang, J., Liu, X. et al. FAM3A Deficiency − Induced Mitochondrial Dysfunction Underlies Post-Infarct Mortality and Heart Failure. J. of Cardiovasc. Trans. Res. 17, 104–120 (2024). https://doi.org/10.1007/s12265-023-10382-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10382-w