Abstract
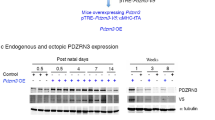
Phosphoinositide-dependent protein kinase-1 (PDK1), a master kinase and involved in multiple signaling transduction, participates in regulating embryonic cardiac development and postnatal cardiac remodeling. Germline PDK1 knockout mice displayed no heart development; in this article, we deleted PDK1 in heart tissue with different cre to characterize the temporospatial features and find the relevance with congenital heart disease(CHD), furthermore to investigate the underlying mechanism. Knocking out PDK1 with Nkx2.5-cre, the heart showed prominent pulmonic stenosis. Ablated PDK1 with Mef2cSHF-cre, the second heart field (SHF) exhibited severe hypoplasia. And deleted PDK1 with αMHC-cre, the mice displayed dilated heart disease, protein analysis indicated PI3K and ERK were activated; meanwhile, PDK1-AKT-GSK3, and S6K-S6 were disrupted; phosphorylation level of Akt473, S6k421/424, and Gsk3α21 enhanced; however, Akt308, S6k389, and Gsk3β9 decreased. In mechanism investigation, we found SHP2 membrane localization and phosphorylation level of SHP2542 elevated, which suggested SHP2 likely mediated the disruption.
Graphical abstract








Similar content being viewed by others
Abbreviations
- PDK1 :
-
Phosphoinositide-dependent protein kinase-1
- CHD:
-
Congenital heart disease
- FHF:
-
First heart field
- SHF:
-
Second heart field
- OFT:
-
Outflow tract
- LV:
-
Left ventricle
- RV:
-
Right ventricle
- VSD:
-
Ventricular septum defect
- ASD:
-
Atrium septum defect
- PI3K:
-
Phosphoinositide-3 kinase
- PIP3:
-
PtdIns(3,4,5)P3
- SHP2 :
-
Src homology-2-containing protein tyrosine phosphatase 2
- PS:
-
Pulmonic stenosis
- PTA:
-
Persistent truncus arteriosus
References
Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41.
Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336:137–44.
Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451:943–8.
Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. 2001;1:435–40.
Plein A, Calmont A, Fantin A, Denti L, Anderson NA, Scambler PJ, Ruhrberg C. Neural crest–derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation. J Clin Investig. 2015;125(7):2661–76.
Harmon AW, Nakano A. Nkx2-5 Lineage tracing visualizes the distribution of second heart field-derived aortic smooth muscle. Genesis. 2013;51:862–9.
Sawada H, Rateri DL, Jessica JM, Mark WM, Alan D. Smooth muscle cells derived from second heart field and cardiac neural crest reside in spatially distinct domains in the media of the ascending aorta-brief report. Arterioscler Thromb Vasc Biol. 2017;37(9):1722–6.
Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7(10):776–89.
Yang KJ, Shin SH, Piao LZ, Shin E, Li YW, Park KA, Byun HS, Won M, Hong JH, Kweon GR, Hur GM, Seok JH, Chun T, Brazil DP, Hemmings BA, Park J. Regulation of 3-phosphoinositide-dependent protein kinase-1 (PDK1) by src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding. J Biol Chem. 2008;283(3):1480–91.
Lauriol J, Jaffre F, Kontaridis MI. The role of the protein tyrosine phosphatase SHP2 in cardiac development and disease. Semin Cell Dev Biol. 2015;37:73–81.
Hof P, Pluskey S, Dhe-Pagganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosinen phosphatase SHP-2. Cell. 1998;92:441–50.
Neel BG, Gu H, Pao L. The ‘Shp’ ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–93.
Araki T, Gordon C, Susan N, Lily M, Roderick TB, Neel BG. Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation PNAS. Proc Natl Acad Sci. 2009;106(12):4736–41.
Moses K, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–80.
Verzi MP, McCulley DJ, Sarah DV, Evdokia D, Brian LB. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–45.
Hou N, Wang J, Li WL, Zhang JS, Yang X. Genotyping analysis of carmyocyte and chondrocyte specific Cre recombinase transgenic mice. Lett Biotechnol. 2005;16(3):262–4.
Yuan B, Wan P, Chu D, Nie J, Cao Y, Luo W. cardiomyocyte-specific Wdr1 knockout demonstrates essential functional roles for actin disassembly during myocardial growth and maintenance in mice. Am J Pathol. 2014;184(20):1967–80.
Lawlor MA, Mora A, Ashby P, William M, Murray-Taits V, Malone L, Prescott A, Lucocq J, Alessi DR. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 2002;21(14):3728–38.
Zhu XX, Shi DY, Li XQ, Gong WJ, Wu FJ, Guo XJ, Xiao H, Liu LX, Zhou H. TLR signalling affects sperm mitochondrial function and motility via phosphatidylinositol 3-kinase and glycogen synthase kinase-3α. Cell Signal. 2016;28(3):148–56.
Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9(13):1654–66.
Zhang L, Aya NK, Nishat S, Cai WB, Cai XQ, Anne MM. Mesodermal Nkx2.5 is necessary and sufficient for early second heart field development. Dev Biol. 2014;390(1):68–79.
Jin HW, Wang HJ, Li J, Yu S, Xu MJ, Qiu ZQ, Xia M, Zhu J, Feng QT, Xie J, Xu B, Yang ZZ. Differential contribution of the two waves of cardiac progenitors and their derivatives to aorta and pulmonary artery. Dev Biol. 2019;450(7):82–9.
Hu J, Shi Y, Xia M, Liu Z, Zhang R, Luo H, Zhang R, Yang ZZ, Yuan B. WDR1 regulated actin dynamics is required for outflow tract and right ventricle development. Dev Biol. 2018;438(2):124–37.
Stefan CM, Tanvi S, Ralston MB, Kelly LB, Brian LB. Cardiovascular development and survival require Mef2c function in the myocardial but not the endothelial lineage. Dev Biol. 2017;445(2):170–7.
Mora A, Davies AM, Bertrand L, Sharif I, Budas GR, Jovanovic S, Mouton VÂ, Kahn CR, Lucocq JM, Gray GA, Jovanovic A, Alessi DR. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBP. 2003;22(18):4666–76.
Feng QT, Di RM, Tao F, Chang Z, Lu SS, Fan WJ, Shan CJ, Li XL, Yang ZZ. PDK1 Regulates vascular remodeling and promotes epithelial-mesenchymal transition in cardiac development. Mol Cell Biol. 2010;30(14):3711–21.
Chang Z, Zhang Q, Feng QT, Xu J, Teng T, Luan Q, Shan C. HuY, Hemmings BA, Gao X, Yang Z, Deletion of Akt1 causes heart defects and abnormal cardiomyocyte proliferation. Dev Biol. 2010;347:384–91.
Krenz M, Gulick J, Osinska HE, Colbert MC, Molkentin JD, Robbins J. Role of ERK1/2 signaling in congenital valve malformations in Noonan syndrome. PNAS. 2008;105(48):18930–5.
Araki T, Mohi MG, Ismat FA, Bronson R, Williams I, Kutok J, Yang WT, Pao I L, Gilliland DG, Epstein J, Neel B. Mouse model of Noonan syndrome reveals cell type– and gene dosage–dependent effects of Ptpn11 mutation. Nat Med. 2004;10(8):849–57.
Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1-/-, S6K2-/- mice exhibit perinatal lethality and rapamycin-sensitive 5-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cel Biol. 2004;24(8):3112–24.
Julie RM, Tetsuo S, Zhang L, Oleg T, Megan CS, Adam L. Deletion of ribosomal S6 kinases does not attenuate pathological, physiological, or insulin-like growth factor 1 receptor-phosphoinositide 3-kinase-induced cardiac hypertrophy. Mol Cell Biol. 2004;24(14):6231–40.
Luo W, Zhao X, Jin H, Tao L, Zhu J, Wang H, Hemmings BA, Yang Z. Akt1 signaling coordinates BMP signaling and beta-catenin activity to regulate second heart field progenitor development. Dev. 2015;142:732–42.
Zhao X, Lu SS, Nie JW, Hu XS, Luo W, Wu XQ, Liu HL, Feng QT, Chang Z, Liu Y, Cao YS, Sun HX, Li XL, Hu YL, Yang ZZ. Phosphoinositide-dependent kinase-1 and mTORC2 synergistically maintain postnatal heart growth and heart function in mice. Mol Cell Biol. 2014;34(11):1966–75.
Edouard T, Combier JP. Functional Effects of PTPN11 (SHP2) Mutations causing LEOPARD syndrome on epidermal growth factor induced phosphoinositide 3-kinase/AKT/glycogen synthase kinase 3 signaling. Mol Cell Biol. 2010;30(10):2498–507.
Zhang SQ, Tsiaras WG, Araki T, Wen GY, Neel BG. Receptor-specific regulation of phosphatidylinositol 3-kinase activation by the protein tyrosine phosphatase SHP2. Mol Cell Biol. 2002;22(12):4062–72.
Maroun CR, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol Cell Biol. 2000;20:8513–25.
Funding
This study was supported by grants from National Natural Science Foundation of China (Nos. 31930029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Nicola Smart oversaw the review of this article.
Clinical Relevance
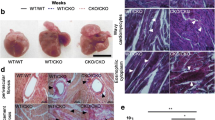
Knocking out PDK1 with Nkx2.5-cre, Mef2cSHF-cre, and αMHC-cre, hearts manifested individual different phenotypes, which are similar to the clinical pulmonic stenosis (PS), persistent truncus arteriosus (PTA), and dilated cardiomyopathy (DCM) respectively; this means PDK1 plays an essential role in embryonic cardiac development and postnatal cardiac remodeling. PS is a common congenital heart disease and accounts for approximately 8% of all congenital heart defects. It includes pulmonary valvular stenosis, infundibular stenosis, and trunk stenosis, which can exist simultaneously or solely, and clinical symptoms may vary from critical stenosis in the new born to mild asymptomatic stenosis without need for therapy throughout life. Knocking out PDK1 with Nkx2.5-cre could induce typical pulmonic stenosis. PTA is a rare severe congenital cardiac disease occupying 0.7–1.4 % of all congenital cardiac malformations. It occurs as a result of the failure of the conotruncal separation during fetal development and is categorized into three subtypes. Half of the clinical cases belong to type I, in which the pulmonary artery arises from lateral aspect of the arterial trunk and then branches into the left and right pulmonary arteries. The truncal valve is usually larger than the normal semilunar valve, number may vary from single to rarely six, and ventricular septal defect is always presented. The phenotype of PDK1 removal with Mef2cSHF-cre mimics persistent truncus arteriosus type I highly. Dilated cardiomyopathy is one of the most common myocardial structural abnormalities associated with heart failure. Knocking out PDK1 with αMHC-cre led to DCM, downregulation of PDK1 and its downstream substrates such as Akt, S6k was the main cause. From this research work, it helps us to understand the pathogenesis of PS, PTA and DCM, and provides some clues for their prevention and diagnosis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, H., Yang, Z., Li, J. et al. Deletion of PDK1 Caused Cardiac Malmorphogenesis and Heart Defects Due to Profound Protein Phosphorylation Changes Mediated by SHP2. J. of Cardiovasc. Trans. Res. 16, 1220–1231 (2023). https://doi.org/10.1007/s12265-023-10380-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10380-y