Abstract
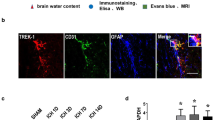
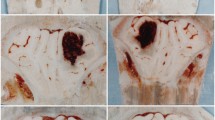
We aimed to select an optimized hematoma expansion (HE) model and investigate the possible mechanism of blood–brain barrier (BBB) damage in mice. The results showed that HE occurred in the group with hypertension combined with hyperglycemia (HH-HE) from 3 to 72 h after intracerebral hemorrhage; this was accompanied by neurological deficits and hardly influenced the survival rate. The receiver operating characteristic curve suggested the criterion for this model was hematoma volume expansion ≥ 45.0%. Meanwhile, HH-HE aggravated BBB disruption. A protector of the BBB reduced HH-HE, while a BBB disruptor induced a further HH-HE. Aquaporin-4 (AQP4) knock-out led to larger hematoma volume and more severe BBB disruption. Furthermore, hematoma volume and BBB disruption were reduced by multiple connexin43 (Cx43) inhibitors in the wild-type group but not in the AQP4 knock-out group. In conclusion, the optimized HE model is induced by hypertension and hyperglycemia with the criterion of hematoma volume expanding ≥ 45.0%. HH-HE leads to BBB disruption, which is dependent on AQP4 and Cx43.






Similar content being viewed by others
References
Van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010, 9: 167–176.
Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol 2012, 11: 307–314.
Chu H, Huang C, Dong J, Yang X, Xiang J, Mao Y, et al. Minimal computed tomography attenuation value within the hematoma is associated with hematoma expansion and poor outcome in intracerebral hemorrhage patients. Neurocrit Care 2019, 31: 455–456.
Chu H, Huang C, Dong J, Yang X, Xiang J, Dong Q, et al. Lactate dehydrogenase predicts early hematoma expansion and poor outcomes in intracerebral hemorrhage patients. Transl Stroke Res 2019, 10: 620–629.
Schlunk F, Van Cott EM, Hayakawa K, Pfeilschifter W, Lo EH, Foerch C. Recombinant activated coagulation factor VII and prothrombin complex concentrates are equally effective in reducing hematoma volume in experimental warfarin-associated intracerebral hemorrhage. Stroke 2012, 43: 246–249.
Na SY, Mracsko E, van Ryn J, Veltkamp R. Idarucizumab improves outcome in murine brain hemorrhage related to dabigatran. Ann Neurol 2015, 78: 137–141.
Zhou W, Zorn M, Nawroth P, Bütehorn U, Perzborn E, Heitmeier S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke 2013, 44: 771–778.
Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997; 28: 1–5.
Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006, 66: 1175–1181.
Morotti A, Charidimou A, Phuah CL, Jessel MJ, Schwab K, Ayres AM, et al. Association between serum calcium level and extent of bleeding in patients with intracerebral hemorrhage. JAMA Neurol 2016, 73: 1285–1290.
Silva Y, Leira R, Tejada J, Lainez JM, Castillo J, Dávalos A. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke 2005, 36: 86–91.
Zheng Y, Hu Q, Manaenko A, Zhang Y, Peng Y, Xu L, et al. 17β-Estradiol attenuates hematoma expansion through estrogen receptor α/silent information regulator 1/nuclear factor-kappa b pathway in hyperglycemic intracerebral hemorrhage mice. Stroke 2015, 46: 485–491.
Chu H, Huang C, Ding H, Gao Z, Dong J, Tang Y, et al. Aquaproin-4 and cerebrovascular diseases. Int J Mol Sci 2016, 17: 1249.
Verkman AS. Knock-out models reveal new aquaporin functions. Handb Exp Pharmacol 2009, 190: 359–381.
Chu H, Tang Y, Dong Q. Protection of vascular endothelial growth factor to brain edema following intracerebral hemorrhage and its involved mechanisms: effect of aquaporin-4. PLoS One 2013, 8: e66051.
Chu H, Ding H, Tang Y, Dong Q. Erythropoietin protects against hemorrhagic blood-brain barrier disruption through the effects of aquaporin-4. Lab Invest 2014, 94: 1042–1053.
Volbers B, Giede-Jeppe A, Gerner ST, Sembill JA, Kuramatsu JB, Lang S, et al. Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology 2018, 90: e1005–e1012.
Yang X, Chu H, Tang Y, Dong Q. The role of connexin43 in hemorrhagic transformation after thrombolysis in vivo and in vitro. Neuroscience 2016, 329: 54–65.
Chu H, Huang C, Gao Z, Dong J, Tang Y, Dong Q. Reduction of ischemic brain edema by combined use of Paeoniflorin and Astragaloside IV via down-regulating connexin 43. Phytother Res 2017, 31: 1410–1418.
Meissner A, Minnerup J, Soria G, Planas AM. Structural and functional brain alterations in a murine model of Angiotensin II-induced hypertension. J Neurochem 2017, 140: 509–521.
Grossetete M, Rosenberg GA. Matrix metalloproteinase inhibition facilitates cell death in intracerebral hemorrhage in mouse. J Cereb Blood Flow Metab 2008, 28: 752–763.
Banks WA, Gray AM, Erickson MA, Salameh TS, Damodarasamy M, Sheibani N, et al. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation 2015, 12: 223.
Zhou Z, Wei X, Xiang J, Gao J, Wang L, You J, et al. Protection of erythropoietin against ischemic neurovascular unit injuries through the effects of connexin43. Biochem Biophys Res Commun 2015, 458: 656–662.
Mracsko E, Javidi E, Na SY, Kahn A, Liesz A, Veltkamp R. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke 2014, 45: 2107–2114.
Zhou W, Schwarting S, Illanes S, Illanes S, Liesz A, Middelhoff M, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke 2011, 42: 3594–3599.
Liew HK, Pang CY, Hsu CW, Hsu CW, Wang MJ, Li TY, et al. Systemic administration of urocortin after intracerebral hemorrhage reduces neurological deficits and neuroinflammation in rats. J Neuroinflammation 2012, 9: 13.
Belayev L, Saul I, Curbelo K, Busto R, Belayev A, Zhang Y, et al. Experimental intracerebral hemorrhage in the mouse histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke 2003, 34: 2221–2227.
Chu H, Xiang J, Wu P, Su J, Ding H, Tang Y, et al. The role of aquaporin 4 in apoptosis after intracerebral hemorrhage. J Neuroinflammation 2014, 11: 184.
Chu H, Tang Y, Dong Q. Protection of granulocyte-colony stimulating factor to hemorrhagic brain injuries and its involved mechanisms: effects of vascular endothelial growth factor and aquaporin-4. Neuroscience 2014, 260: 59–72.
Huang C, Dai C, Gong K, Zuo H, Chu H. Apelin-13 protects neurovascular unit against ischemic injuries through the effects of vascular endothelial growth factor. Neuropeptides 2016, 60: 67–74.
Chu H, Yang X, Huang C, Gao Z, Tang Y, Dong Q. Apelin-13 Protects against ischemic blood-brain barrier damage through the effects of aquaporin-4. Cerebrovasc Dis 2017, 44: 10–25.
Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 2016, 375: 1033–1043.
Liu J, Gao BB, Clermont AC, Blair P, Chilcote TJ, Sinha S, et al. Hyperglycemia-induced cerebral hematoma expansion is mediated by plasma kallikrein. Nat Med 2011, 17: 206–210.
Delcourt C, Huang Y, Wang J, Heeley E, Lindley R, Stapf C, et al. The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2). Int J Stroke 2010, 5: 110–116.
Dong J, Yang X, Xiang J, Dong Q, Tang Y, Chu H. Hypodensities detected at 1.5–3 h after intracerebral hemorrhage better predicts secondary neurological deterioration. J Neurol Sci 2019, 396: 219–224
Hu HM, Li B, Wang XD, Guo YS, Hui H, Zhang HP, et al. Fluoxetine is neuroprotective in early brain injury via its anti-inflammatory and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Neurosci Bull 2018, 34:951–962.
Schlunk F, Greenberg SM. The pathophysiology of intracerebral hemorrhage formation and expansion. Transl Stroke Res 2015, 6:257–263.
Boulouis G, Dumas A, Betensky RA, Brouwers HB, Fotiadis P, Vashkevich A, et al. Anatomic pattern of intracerebral hemorrhage expansion: relation to CT angiography spot sign and hematoma center. Stroke 2014, 45:1154–1156.
Freeze WM, Jacobs HIL, Schreuder FHBM, van Oostenbrugge RJ, Backes WH, Verhey FR, et al. Blood-brain barrier dysfunction in small vessel disease related intracerebral hemorrhage. Front Neurol 2018, 9: 926.
Howe MD, Zhu L, Sansing LH, Gonzales NR, McCullough LD, Edwards NJ. Serum markers of blood-brain barrier remodeling and fibrosis as predictors of etiology and clinicoradiologic outcome in intracerebral hemorrhage. Front Neurol 2018, 9: 746.
Urrutia A, Rubio-Araiz A, Gutierrez-Lopez MD, ElAli A, Hermann DM, O’Shea E, et al. A study on the effect of JNK inhibitor, SP600125, on the disruption of blood-brain barrier induced by methamphetamine. Neurobiol Dis 2013, 50: 49–58.
Yu Y, Xue BJ, Zhang ZH, Wei SG, Beltz TG, Guo F, et al. Early interference with p44/42 mitogen-activated protein kinase signaling in hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension. Hypertension 2013, 61: 842–849.
Farrokhnia N, Roos MW, Terént A, Lennmyr F. Differential early mitogen-activated protein kinase activation in hyperglycemic ischemic brain injury in the rat. Eur J Clin Invest 2005, 35: 457–463.
Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, et al. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J 2007, 21: 108–116.
Nico B, Frigeri A, Nicchia GP, Quondamatteo F, Herken R, Errede M, et al. Role of aquaporin-4 water channel in the development and integrity of the blood-brain barrier. J Cell Sci 2001, 114: 1297–1307.
Kimura A, Hsu M, Seldin M, Verkman AS, Scharfman HE, Binder DK. Protective role of aquaporin-4 water channels after contusion spinal cord injury. Ann Neurol 2010, 67: 794–801.
Uzu M, Sin WC, Shimizu A, Sato H. Conflicting roles of connexin43 in tumor invasion and growth in the central nervous system. Int J Mol Sci 2018, 11: 19.
Chew SSL, Johnson CS, Green CR, Danesh-Meyer HV. Role of connexin43 in central nervous system injury. Exp Neurol 2010, 225: 250–261.
Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J 2009, 419: 261–272.
Ezan P, André P, Cisternino S, Saubaméa B, Boulay AC, Doutremer S, et al. Deletion of astroglial connexins weakens the blood-brain barrier. J Cereb Blood Flow Metab 2012, 32: 1457–1467.
Lima F, Niger C, Hebert C, Stains JP. Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/runx2-dependent mechanism. Mol Biol Cell 2009, 20: 2697–2708.
McCoy ES, Haas BR, Sontheimer H. Water permeability through aquaporin-4 is regulated by protein kinase C and becomes rate-limiting for glioma invasion. Neuroscience 2010, 168: 971–981.
Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP. ERK acts in parallel to PKC delta to mediate the connexin43-dependent potentiation of Runx2 activity by FGF2 in MC3T3 osteoblasts. Am J Physiol Cell Physiol 2012, 302: C1035–C1044.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81500998 and 81901102), the Science and Technology Commission of Shanghai Municipality (16140903200), Shanghai Sixth People’s Hospital Medical Group (2017LY01), the Research Fund of Shanghai Tongren Hospital, Shanghai Jiaotong University School of Medicine (TRYJ201701), and the Research Fund of North Huashan Hospital, Fudan University (HSBY2019004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chu, H., Gao, Z., Huang, C. et al. Relationship Between Hematoma Expansion Induced by Hypertension and Hyperglycemia and Blood–brain Barrier Disruption in Mice and Its Possible Mechanism: Role of Aquaporin-4 and Connexin43. Neurosci. Bull. 36, 1369–1380 (2020). https://doi.org/10.1007/s12264-020-00540-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-020-00540-4