Summary
In the field of thrombosis and hemostasis the focus was laid on the prophylaxis and treatment of venous thromboembolism in patients with active cancer during this years’ meeting of the American Society of Hematology (ASH) in San Diego. The Adam-VTE trial completed the series of studies using Xa-antagonists for the treatment of cancer-associated thromboembolism. Apixaban reduced the number of recurrent thromboembolic events compared to treatment with dalteparin without increasing the bleeding risk. Furthermore, the use of a prophylactic dose of rivaroxaban or apixaban showed positive results in the primary prevention of venous thromboembolism in ambulatory cancer patients being at high risk for thromboembolic events. A safe standardized perioperative management for direct oral anticoagulants was also presented during the meeting in noncancer patients with atrial fibrillation. This short review will summarize the most clinically relevant highlights in the field of thrombosis and hemostasis of the ASH meeting 2018.
Similar content being viewed by others
-
Xa-antagonists can be used for treatment for cancer-associated thrombosis and pulmonary embolism
-
A prophylactic dose of apixaban as well as rivaroxaban is safe for VTE prophylaxis in cancer patients with high risk for thromboembolic events
-
No need for bridging of direct oral anticoagulants with LMWH by using a standardized perioperative management
Introduction
Cancer patients have an increased risk of suffering from venous thromboembolism (VTE) compared to noncancer patients. Several factors, besides the tumor, like prothrombotic chemotherapy, immobilization and the use of central venous catheters lead to the higher risk for thromboembolic events [1]. Anticoagulant treatment of cancer-associated VTE can be challenging due to risk of bleeding from the tumor itself as well as possible drug–drug interaction or thrombocytopenia.
During this years’ ASH meeting the data for the use of direct oral anticoagulants (DOAC), specifically the Xa-antagonists, as a treatment option for cancer-associated VTE were completed with the presentation of the data for apixaban in the Adam-VTE trial [2]. However, another topic was the primary prevention of VTEs in cancer patients being at high risk for thromboembolic events using a prophylactic dose of rivaroxaban [3, 4] or apixaban [5]. Finally, a prospective trial in noncancer patients with atrial fibrillation who are taking DOACs was presented to test the risk of bleeding and thromboembolic events using a standardized perioperative management [6].
Treatment of venous thromboembolism associated with cancer
Since the publication of the Clot Study in 2003 it has almost become a dogma that low molecular weight heparins (LMWH) are the preferred treatment of choice in cancer-associated VTE [7]. Subsequent studies using different LMWHs supported the concept that LMWH can reduce the risk of VTE recurrence compared to treatment with vitamin K antagonists, albeit to different degrees. For the first time the Hokusai-VTE Cancer trial (edoxaban) and the Select-D trial (rivaroxaban), which were both already presented during the ASH meeting 2017 in Atlanta, compared the treatment of oral Xa antagonists to LMWH in a prospective trial specifically in patients suffering from active cancer and VTE [8, 9]. In both studies, the results did not show inferiority in the composite outcome of recurrent thromboembolism or major bleeding. However, recurrent thromboembolism was less likely in patients treated with DOACs, whereas major bleeding and clinically relevant nonmajor bleeding (CRNMB) occurred more often using the oral anticoagulants. Especially in patients with cancer of the upper gastrointestinal tract, the number of bleedings increased when the patients were treated with rivaroxaban or edoxaban, respectively [8, 9].
McBane et al. presented the data of the comparison of apixaban and dalteparin in the Adam-VTE Trial during the ASH meeting [2]. A total of 287 patients with cancer-associated acute VTE were randomly assigned to receive either apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily or subcutaneous dalteparin (200 IU/kg for 1 month followed by 150 IU/kg once daily) for 6 months. Major bleeding did not occur in the apixaban group whereas 3 of the 145 patients (2.1%) in the dalteparin group had major bleedings. Major bleeding plus CRNMB were similar at 9% for both groups. Recurrent VTE occurred in 5 patients (3.4%) in the apixaban group and 20 patients (14.1%) in the dalteparin group (p = 0.0182). There were no differences in mortality comparing the apixaban (15.9%) and dalteparin (10.6%) groups at 6 months (hazard ratio [HR] 1.36, 95% confidence interval [CI] 0.79–2.35).
With regard to the already published data for rivaroxaban and edoxaban [8, 9], these data support the clinical utility of oral Xa antagonists for the acute treatment of VTE in cancer patients. However, caution is still advised when used in patients with gastrointestinal tract malignancies, especially upper gastrointestinal tract malignancies.
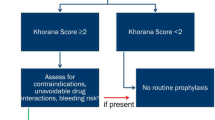
Thromboprophylaxis in high-risk ambulatory cancer patients
Thromboprophylaxis with LMWH, unfractionated heparin or fondaparinux in hospitalized surgical and medical cancer patients with reduced mobility is recommended [10], whereas the general role of primary pharmacological prophylaxis in ambulatory cancer patients is unclear. Different risk assessment models have been established to identify cancer patients at high risk for VTE ([11,12,13]; Table 1). VTE prophylaxis in ambulatory cancer patients with LMWH using either a prophylactic or intermediate therapeutic dose was assessed in general cancer patients at high risk (Khorana score ≥3) or in specific cancer entities [14, 15]. In line with the largest available meta-analysis [16], LMWH prophylaxis can significantly reduce the number of VTEs compared to no prophylaxis, but increases the bleeding rate and does not affect overall survival. Therefore, thrombophrophylaxis in the ambulatory setting is so far only recommended for patients with locally advanced or metastatic pancreatic cancer and myeloma patients treated with immunomodulatory drugs (IMIDs) in combination with another systemic anticancer therapy [10].
During the ASH Meeting 2018 Alok Khorana presented the data of the CASSINI trial as the Late Breaking Abstract–1 [3], and the study has been subsequently published [4]. The prophylaxis with rivaroxaban 10 mg once daily was compared to placebo in the double-blind, randomized, multicenter study in adult ambulatory patients with various cancers at increased risk for VTE (defined as Khorana score ≥2). In all, 1080 patients were screened with lower extremity ultrasounds for deep-vein thrombosis (DVT) before the enrollment in the study and every 8 weeks during the observation period of 180 days. A total of 49 (4.53%) cancer patients had DVT on baseline screening, and 841 patients were finally randomized. Finally, 25 of 420 patients (5.95%) and 37 of 421 patients (8.79%) (HR, 0.66; p = 0.101) in the rivaroxaban and placebo groups, respectively, developed a VTE in the up-to-day 180 observation period. Notably, 38.7% experienced events after discontinuing the study drug. During the on-treatment period, 11 of 420 patients (2.62%) and 27 of 421 (6.41%) developed a VTE in rivaroxaban and placebo groups, respectively. Major bleeding or CRNMB occurred in 1.98 and 2.72% of the patients in the rivaroxaban group and 0.99 and 1.98% in the placebo group. There was no significant difference in the all-cause mortality between the two groups; however the composite of the occurrence of DVT with all-cause mortality occurred in 23.1% of patients in rivaroxaban group and 29.5% in placebo group (p = 0.03) [3, 4].
Carrier et al. published similar data for the VTE prophylaxis with apixaban in ambulatory cancer patients with intermediate-to-high risk for VTE (Khorana score ≥2) in the AVERT trial [5]; however these data were not presented during the ASH meeting 2018. In summary, 563 patients were included in the analysis of the study. Overall, 12 of 288 patients (4.2%) developed a VTE in the apixaban group and 28 of 275 patients (10.2%) in the placebo group, respectively (HR 0.41; P < 0.001). During the treatment period, major bleeding occurred in 6 apixaban-treated patients (2.1%), while only 3 patients (1.1%) suffered from major bleedings in the placebo arm. In all, 35 patients (12.2%) died in the apixaban group and 27 patients (9.8%) in the placebo group. Of the 62 deaths, 54 (87%) were related to cancer or cancer progression.
In summary, the Khorana risk score is an established tool to identify cancer patients with a high risk of VTE [11]. A prophylactic dose of apixaban and rivaroxaban significantly reduced VTE, and in general, the incidence of major bleeding was low in this high risk patient cohort [3,4,5]. In conclusion, a cut-off for the Khorana score of ≥2 may be implemented in future recommendations regarding thromboprophylaxis in at-risk ambulatory cancer patients, even though the bleeding risk has to be taken into account.
Direct oral anticoagulants and perioperative management
Perioperative procedure in patients taking DOACs are mainly based on pharmacokinetic studies ([17]; Table 2). Patients’ characteristics (kidney function, CHA2DS2-VASc score, history of previous thromboembolic events) as well as the bleeding risk of the planned intervention have to be taken into account when determining if and when the anticoagulant treatment needs to be temporary discontinued. Preoperative bridging with LMWH is generally not recommended in patients treated with DOACs [17].
In another late breaking abstract Douketis et al. presented the data of the prospective the Perioperative Anticoagulant Use for Surgery Evaluation (PAUSE) study [6]. In this international trial 3007 patients from 23 sites were enrolled in 3 parallel DOAC cohorts of patients with atrial fibrillation taking apixaban (n = 1257), dabigatran (n = 668) or rivaroxaban (n = 1082) and requiring anticoagulant interruption for an elective surgery/procedure. DOACs were interrupted for 1 day before and after surgery for a low bleed risk surgery and 2 days before and after a high bleed surgery; longer cessation was only done in patients on dabigatran depending on the kidney function (CrCl <50 mL/min). In all, 3.5% of the interventions were defined as high bleeding risk surgery/procedures. It has to be mentioned that peri- and postoperative thromboprophylaxis with LMWH was allowed during the trial. Using the standardized approach, the 30-day postoperative rate of bleedings and arterial thromboembolism was generally low. In 2541 (84.5%) patients, preoperative DOAC levels were measured. Most of the patients (>90% overall; 98.8% at high bleeding risk) had a minimal or no residual DOAC level at the time of the surgery/procedure. In summary, the prospective PAUSE study showed for the first time that bleeding-risk adapted temporary cessation without LMWH bridging is safe in patients with DOACs and atrial fibrillation undergoing invasive procedures. Importantly, these data might not be transferable to patients with a history of deep vein thrombosis or pulmonary embolism or high risk thrombophilia, such as antiphospholipid syndrome.
Conclusion
The knowledge regarding the use of DOACs is still increasing. On the one hand, in cancer patients oral Xa antagonists have proven to be safe in the treatment of cancer-associated VTEs. Furthermore, apixaban and rivaroxaban can be used for VTE prophylaxis in selected ambulatory high-risk cancer patients to reduce thromboembolic events. On the other hand, the PAUSE study showed in a prospective trial that in patients with atrial fibrillation temporary discontinuation of DOACs without LMWH-bridging is safe for patients undergoing invasive procedures.
Abbreviations
- ASH:
-
American Society of Hematology
- CRNMB:
-
Clinical relevant nonmajor bleedings
- DOAC:
-
Direct oral anticoagulants
- DVT:
-
Deep vein thrombosis
- IMIDs:
-
Immunomodulatory drugs
- LMWH:
-
Low molecular weight heparin
- PE:
-
Pulmonary embolism
- VKA:
-
Vitamin K antagonists
- VTE:
-
Venous thromboembolism
References
Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–46.
McBane RD, Wysokinski WE, Le-Rademacher J, et al. Apixaban, Dalteparin, in active cancer associated venous Thromboembolism, the ADAM VTE trial. Blood. 2018;132:421. https://doi.org/10.1182/blood-2018-99-118808.
Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban Thromboprophylaxis in high-risk ambulatory cancer patients receiving systemic therapy: results of a randomized clinical trial (CASSINI). Blood. 2018;132:LBA-1. https://doi.org/10.1182/blood-2018-120738.
Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for Thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380(8):720–8. https://doi.org/10.1056/NEJMoa1814630.
Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous Thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711–9. https://doi.org/10.1056/NEJMoa1814468.
Douketis J, Spyropoulos AC, Duncan JM, et al. Perioperative anticoagulant use for surgery evaluation (PAUSE) study: a Perioperative management plan for patients with atrial fibrillation who are receiving a direct oral anticoagulant. Blood. 2018;132:LBA-5. https://doi.org/10.1182/blood-2018-120770.
Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–53.
Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous Thromboembolism. N Engl J Med. 2018;378(7):615–24. https://doi.org/10.1056/NEJMoa1711948.
Young AM, Marshall A, Thirlwall J, Chapman O, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous Thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017–23. https://doi.org/10.1200/JCO.2018.78.8034.
Farge D, Bounameaux H, Brenner B, et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016;17(10):e452–e66. https://doi.org/10.1016/S1470-2045(16)30369-2.
Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–7. https://doi.org/10.1182/blood-2007-10-116327.
Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–82. https://doi.org/10.1182/blood-2010-02-270116.
Verso M, Agnelli G, Barni S, et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7(3):291–2. https://doi.org/10.1007/s11739-012-0784-y.
Khorana AA, Francis CW, Kuderer NM, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: A randomized trial. Thromb Res. 2017;151:89–95. https://doi.org/10.1016/j.thromres.2017.01.009.
Ek L, Gezelius E, Bergman B, et al. Randomized phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: the RASTEN trial. Ann Oncol. 2018;29(2):398–404. https://doi.org/10.1093/annonc/mdx716.
Di Nisio M, Porreca E, Candeloro M, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2016;12:CD8500. https://doi.org/10.1002/14651858.CD008500.pub4.
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93. https://doi.org/10.1093/eurheartj/ehy136.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Feistritzer declares that he has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Notice:
The licensing of the DOAC may vary according to the local authorities.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Feistritzer, C. Update in thrombosis and hemostasis: ASH meeting 2018. memo 12, 212–215 (2019). https://doi.org/10.1007/s12254-019-0491-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-019-0491-8