Abstract
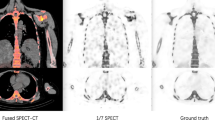
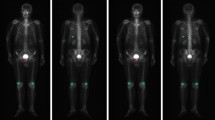
To improve image quality for low-count bone scintigraphy using deep learning and evaluate their clinical applicability. Six hundred patients (training, 500; validation, 50; evaluation, 50) were included in this study. Low-count original images (75%, 50%, 25%, 10%, and 5% counts) were generated from reference images (100% counts) using Poisson resampling. Output (DL-filtered) images were obtained after training with U-Net using reference images as teacher data. Gaussian-filtered images were generated for comparison. Peak signal-to-noise ratio (PSNR) and structural similarity (SSIM) to the reference image were calculated to determine image quality. Artificial neural network (ANN) value, bone scan index (BSI), and number of hotspots (Hs) were computed using BONENAVI analysis to assess diagnostic performance. Accuracy of bone metastasis detection and area under the curve (AUC) were calculated. PSNR and SSIM for DL-filtered images were highest in all count percentages. BONENAVI analysis values for DL-filtered images did not differ significantly, regardless of the presence or absence of bone metastases. BONENAVI analysis values for original and Gaussian-filtered images differed significantly at ≦25% counts in patients without bone metastases. In patients with bone metastases, BSI and Hs for original and Gaussian-filtered images differed significantly at ≦10% counts, whereas ANN values did not. The accuracy of bone metastasis detection was highest for DL-filtered images in all count percentages; the AUC did not differ significantly. The deep learning method improved image quality and bone metastasis detection accuracy for low-count bone scintigraphy, suggesting its clinical applicability.






Similar content being viewed by others
References
Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
Maffioli L, Florimonte L, Pagani L, Butti I, Roca I. Current role of bone scan with phosphonates in the follow-up of breast cancer. Eur J Nucl Med Mol Imaging. 2004;31:S143–8.
Govaert GAM, Glaudemans AWJM. Nuclear medicine imaging of posttraumatic osteomyelitis. Eur J Trauma Emerg Surg. 2016;42:397–410.
Abdelrazek S, Szumowski P, Rogowski F, Kociura-Sawicka A, Mojsak M, Szorc M. Bone scan in metabolic bone diseases Review. Nucl Med Rev Cent East Eur. 2012;15:124–31.
Koppula BR, Morton KA, Al-Dulaimi R, Fine GC, Damme NM, Brown RKJ. SPECT/CT in the evaluation of suspected skeletal pathology. Tomography. 2021;7:581–605.
Saha S, Burke C, Desai A, Vijayanathan S, Gnanasegaran G. SPECT-CT: applications in musculoskeletal radiology. Br J Radiol. 2013;86:20120519.
Zhang L, He Q, Zhou T, et al. Accurate characterization of 99mTc-MDP uptake in extraosseous neoplasm mimicking bone metastasis on whole-body bone scan: contribution of SPECT/CT. BMC Med Imaging. 2019;19:44.
Pan B, Qi N, Meng Q, et al. Ultra high speed SPECT bone imaging enabled by a deep learning enhancement method: a proof of concept. EJNMMI Phys. 2022;9:43.
Pain CD, Egan GF, Chen Z. Deep learning-based image reconstruction and post-processing methods in positron emission tomography for low-dose imaging and resolution enhancement. Eur J Nucl Med Mol Imaging. 2022;49:3098–118.
Cheng Z, Wen J, Huang G, Yan J. Applications of artificial intelligence in nuclear medicine image generation. Quant Imaging Med Surg. 2021;11:2792–822.
Arabi H, Zaidi H. Applications of artificial intelligence and deep learning in molecular imaging and radiotherapy. Eur J Hybrid Imaging. 2020;4:17.
Nensa F, Demircioglu A, Rischpler C. Artificial intelligence in nuclear medicine. J Nucl Med. 2019;60:29S-37S.
Shao W, Rowe SP, Du Y. Artificial intelligence in single photon emission computed tomography (SPECT) imaging: a narrative review. Ann Transl Med. 2021;9:820.
Decuyper M, Maebe J, Van Holen R, Vandenberghe S. Artificial intelligence with deep learning in nuclear medicine and radiology. EJNMMI Phys. 2021;8:81.
Zhang D, Pretorius PH, Lin K, et al. A novel deep-learning-based approach for automatic reorientation of 3D cardiac SPECT images. Eur J Nucl Med Mol Imaging. 2021;48:3457–68.
Ito T, Maeno T, Tsuchikame H, et al. Adapting a low-count acquisition of the bone scintigraphy using deep denoising super-resolution convolutional neural network. Phys Med. 2022;100:18–25.
Sadik M, Hamadeh I, Nordblom P, et al. Computer-assisted interpretation of planar whole-body bone scans. J Nucl Med. 2008;49:1958–65.
Sadik M, Suurkula M, Höglund P, Järund A, Edenbrandt L. Improved classifications of planar whole-body bone scans using a computer-assisted diagnosis system: a multicenter, multiple-reader, multiple-case study. J Nucl Med. 2009;50:368–75.
Sadik M, Jakobsson D, Olofsson F, Ohlsson M, Suurkula M, Edenbrandt L. A new computer-based decision-support system for the interpretation of bone scans. Nucl Med Commun. 2006;27:417–23.
White D, Lawson RS. A Poisson resampling method for simulating reduced counts in nuclear medicine images. Phys Med Biol. 2015;60:N167–76.
Belhocine T, Rachinsky I, Akincioglu C, et al. How Useful is an integrated SPECT/CT in clinical setting and research?: evaluation of a low radiation dose 4 slice system. Open Medical Imaging J. 2008;2:80–108.
Vanhove C, Franken PR, Defrise M, Deconinck F, Bossuyt A. Reconstruction of gated myocardial perfusion SPET incorporating temporal information during iterative reconstruction. Eur J Nucl Med Mol Imaging. 2002;29:465–72.
Minarik D, Enqvist O, Trägårdh E. Denoising of scintillation camera images using a deep convolutional neural network: a monte carlo simulation approach. J Nucl Med. 2020;61:298–303.
Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. arXiv: 1505.04597
Liu CC, Qi J. Higher SNR PET image prediction using a deep learning model and MRI image. Phys Med Biol. 2019;64:115004.
Chen KT, Gong E, de Carvalho Macruz FB, et al. Ultra-low-dose 18F-florbetaben amyloid pet imaging using deep learning with multi-contrast MRI inputs. Radiology. 2019;290:649–56.
Lu W, Onofrey JA, Lu Y, et al. An investigation of quantitative accuracy for deep learning based denoising in oncological PET. Phys Med Biol. 2019;64:165019.
Ardenfors O, Svanholm U, Jacobsson H, Sandqvist P, Grybäck P, Jonsson C. Reduced acquisition times in whole body bone scintigraphy using a noise-reducing Pixon®-algorithm-a qualitative evaluation study. EJNMMI Res. 2015;5:48.
Kovacs A, Bukki T, Légrádi G, et al. Robustness analysis of denoising neural networks for bone scintigraphy. Nucl Instrum Methods Phys Res Sect A. 2022;1039: 167003.
Liu S, Feng M, Qiao T, et al. Deep learning for the automatic diagnosis and analysis of bone metastasis on bone scintigrams. Cancer Manag Res. 2022;14:51–65.
Acknowledgements
We would like to thank Editage (https://www.editage.com/) for editing and reviewing this manuscript for English language.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
No conflicts of interest.
Research involving human participants
All procedures involving human participants performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The institutional review board approved this retrospective study and waived the requirement for informed consent from the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Murata, T., Hashimoto, T., Onoguchi, M. et al. Verification of image quality improvement of low-count bone scintigraphy using deep learning. Radiol Phys Technol 17, 269–279 (2024). https://doi.org/10.1007/s12194-023-00776-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12194-023-00776-5