Abstract
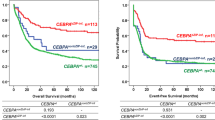
Deregulated mitochondrial metabolism and biogenesis have been studied in acute myeloid leukemia (AML); yet, the relevance of mitochondrial-encoded gene expression on AML outcomes is unknown. This study was conducted to assess clinical significance of expression of mitochondrial-encoded genes, namely ND3, SDHB, Cytochrome b, Cytochrome C, and ATP6, in pediatric AML. Pediatric AML patients from July 2013 to June 2016 were enrolled in this prospective study. Relative genes expression was determined using real-time PCR, and expressed as fold change. 123 AML patients were enrolled, median age 10 (range 0.7–18 years). ND3 gene expression was significantly increased in poor-risk cytogenetics (P = 0.03). In univariate analysis, high ND3 and ATP6 gene expression was significantly associated with inferior EFS (P = 0.01 and P = 0.04, respectively) and OS (P = 0.02 and P = 0.01, respectively), whereas, in multivariate analysis, ND3 gene expression emerged as the only independent prognostic factor for EFS and OS (P = 0.04 and P = 0.03). ND3 gene expression is a significant predictor of EFS and OS in pediatric AML, and should be evaluated as a potential biomarker.



Similar content being viewed by others
References
Wallace DC. Diseases of the mitochondrial DNA. Annu Rev Chem Biomol Eng. 1992;61:1175–212.
Grossman L. Mitochondrial DNA mutations and human disease. Environ Mutagen. 1995;25:30–7.
Larsson NG, Luft R. Revolution in mitochondrial medicine. FEBS Lett. 1999;455:199–202.
Grossman L, Shoubridge E. Mitochondrial genetics and human disease. BioEssays. 1996;18:983–91.
Weiss H, Friedrich T, Hofhaus G, Preis D. The respiratory-chain NADH dehydrogenase (complex 1) of mitochondria. Eur J Biochem. 1991;197:563–76.
Hatefi Y. The mitochondrial phosphorylation system and oxidative phosphorylation. Annu Rev Biochem. 1985;54:1015–69.
Robinson BH. Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the defect. Biochim Biophys Acta. 1998;1364:271–86.
Carew JS, Huang P. Mitochondrial defects in cancer. Mol Cancer. 2002;1:1–12.
Fliss MS, Usadel H, Cobarello OL. Facile detection of mitochondrial DNA mutations in tumours and bodily fluids. Science. 2000;287:2017–9.
Bianchi MS, Bianchi NO, Bailliet G. Mitochondrial DNA mutations in normal and tumor tissues from breast cancer patients. Cytogenet Cell Genet. 1995;71:99–103.
Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat Genet. 1998;20:291–3.
Horton TM, Petros JA, Heddi A, Shoffner J, Kaufman AE, Graham SD Jr, et al. Novel mitochondrial DNA deletion found in a renal cell carcinoma. Genes Chromosomes Cancer. 1996;15:95–101.
Luciakovak KS. Increased steady-state levels of several mitochondrial and nuclear gene transcripts in rat hepatoma with a low content of mitochondria. Eur J Biochem. 1992;205:1187–93.
Tamura G, Nishizuka S, Maesawa C, Suzuki Y, Lwaya T, Sakata K, et al. Mutations in mitochondrial control region DNA in gastric tumours of Japanese patients. Eur J Cancer. 1999;35:316–9.
Clayton DA, Vinograd J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature. 1967;216:652–7.
LaBiche RA, Yoshida M, Gallick GE, Irimura T, Robberson DL, Klostergaard J, et al. Gene expression and tumor cell escape from host effector mechanisms in murine large cell lymphoma. J Cell Biochem. 1988;36:393–403.
Sharp MGF, Adams SM, Walker RA, Brammar WJ, Varley JM. Differential expression of the mitochondrial gene cytochrome oxidase II in benign and malignant breast tissue. J Pathol. 1992;168:163–8.
Lu X, Walker T, Macmanus JP, Walker T, Macmanus JP, Seligy VL. Differentiation of HT-29 human colonic adenocarcinoma cells correlates with increased expression of mitochondrial RNA: effects of trehalose on cell growth and maturation differentiation of HT-29 human colonie adenocarcinoma cells correlates with increase. Cancer Res. 1992;37:18–25.
Reznik E, Miller ML, Şenbabaoğlu Y, Riaz N, Sarungbam J, Tickoo SK, et al. Mitochondrial DNA copy number variation across human cancers. ELife. 2016;5:1–20.
Reznik ED, Wang Q, La K, Schultz N, Sander C. Mitochondrial respiratory gene expression is suppressed in many cancers. ELife. 2017;6:1–16.
Schildgen V, Wulfert M, Gattermann N. Impaired mitochondrial gene transcription in myelodysplastic syndromes and acute myeloid leukemia with myelodysplasia-related changes. Exp Hematol. 2011;39:666–75.
Tyagi A, Pramanik R, Vishnubhatla S, Ali S, Bakhshi R, Chopra A, et al. Pattern of mitochondrial D-loop variations and their relation with mitochondrial encoded genes in pediatric acute myeloid leukemia. Mutat Res. 2018;810:13–8.
Shaffer LG, McGowan-Jordan J, Schmid M. An international system for human cytogenetic nomenclature (ISCN). Basel: Karger; 2013. p. 88–95.
Chopra A, Soni S, Pati H, Kumar D, Diwedi R, Verma D, et al. Nucleophosmin mutation analysis in acute myeloid leukaemia: immunohistochemistry as a surrogate for molecular techniques. Indian J Med Res. 2016;143:763–8.
Sharawat SK, Raina V, Kumar L, Sharma A, Bakhshi R, Vishnubhatla S, et al. High fms-like tyrosine kinase-3 (FLT3) receptor surface expression predicts poor outcome in FLT3 internal tandem duplication (ITD) negative patients in adult acute myeloid leukaemia: a prospective pilot study from India. Indian J Med Res. 2016;143:S11–S16.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from international panel. Blood. 2017;129:424–48.
Burnett AK, Russell NH, Hills RK, Kell J, Cavenagh J, Kjeldsen L, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2016;125:3878–86.
Ahmad F, Mandava S, Das BR. Analysis of FLT3-ITD and FLT3-Asp835 mutations in de novo acute myeloid leukemia: evaluation of incidence, distribution pattern, correlation with cytogenetics and characterization of internal tandem duplication from Indian population. Cancer Investig. 2010;28:63–73.
Wulfert M, Küpper AC, Tapprich C, Bottomley SS, Bowen D, Germing U, et al. Analysis of mitochondrial DNA in 104 patients with myelodysplastic syndromes. Exp Hematol. 2008;36:577–86.
Chen ML, Logan TD, Hochberg ML, Shelat SG, Yu X, Wilding GE, et al. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood. 2009;114:4045–53.
Sotgia F, Lisanti MP. Mitochondrial markers predict survival and progression in non-small cell lung cancer (NSCLC) patients: use as companion diagnostics. Oncotarget. 2017;8:68095–107.
Sotgia F, Fiorillo M, Lisanti MP. Mitochondrial markers predict recurrence, metastasis and tamoxifen-resistance in breast cancer patients: early detection of treatment failure with companion diagnostics. Oncotarget. 2017;8:68730–45.
Selvanayagam P, Rajaraman S. Detection of mitochondrial genome depletion by a novel cDNA in renal cell carcinoma. Lab Investig. 1996;74:592.
Wong TWL, Yu HY, Kong SK, Fung KP, Kwok TT. The decrease of mitochondrial NADH dehydrogenase and drug induced apoptosis in doxorubicin resistant A431 cells. Life Sci. 2000;67:1111–8.
Acknowledgements
We thank our nursing staff, data entry operator, patients and their parents who participated in the study. We also acknowledge the following funding agency 1. AIIMS, (Grant no. F.5-59/IRG/2010/RS) Intramural Grant.
Author information
Authors and Affiliations
Contributions
AT and SB designed the study; AT, RB, and AS contribute to acquisition and interpretation of data; SV was the statistician, analyzed and interpreted the results; AT, RP, and SB wrote the paper. All authors reviewed and gave the final approval for the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Approval for the study was taken from the Institute Ethical Committee vide letter number: IEC/NP-336/2012.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tyagi, A., Pramanik, R., Bakhshi, R. et al. Expression of mitochondrial genes predicts survival in pediatric acute myeloid leukemia. Int J Hematol 110, 205–212 (2019). https://doi.org/10.1007/s12185-019-02666-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-019-02666-2