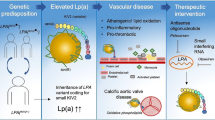
Abstract
Purpose of Review
Lipoprotein(a) [Lp(a)] is a pro-inflammatory, pro-thrombotic, and pro-atherogenic lipoprotein particle. Lp(a) binds and transports oxidized phospholipids in the bloodstream. It is one of the strongest genetic risk factors for coronary artery disease, stroke, and calcific aortic valve stenosis.
Recent Findings
Elevated Lp(a) levels, or hyperlipoproteinemia(a), is associated with cardiovascular outcomes even in high-risk individuals who achieve their low-density lipoprotein cholesterol target with statins. Lifestyle modification therapy and dietary supplements have little impact on plasma Lp(a) levels. However, in individuals with hyperlipoproteinemia(a), the adherence to ideal cardiovascular health metrics (not smoking, having a healthy diet, being physically active, having a normal body mass index, having a normal blood pressure as well as blood sugar and cholesterol levels) might reduce the cardiovascular risk associated with elevated Lp(a) levels. Cardiovascular drugs such as proprotein convertase subtilisin/kexin type 9 inhibitors and niacin provide modest reductions in Lp(a) levels. Lp(a)-targeted therapies are currently being developed, and their impact on cardiovascular risk is yet to be determined.
Summary
In the absence of approved Lp(a)-targeted therapies, a holistic approach combining strict control of traditional cardiovascular risk factors and the adequate management of lifestyle-related risk factors is likely to significantly reduce cardiovascular risk in patients with hyper-Lp(a).

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI working group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71:177–92 This statement from the NHLBI working group on Lp(a) provides an excellent overview of the current status of Lp(a) research, knowledge gaps and future research that should be performed to better understand the pathobiology and clinical relevance of hyper-Lp(a).
Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J Lipid Res. 2013;54:2815–30.
Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273:6–30.
Rao JF, Schork XA, Maihofer MA, et al. Heritability of biomarkers of oxidized lipoproteins: twin pair study. Arterioscler Thromb Vasc Biol. 2015;35:1704–11.
Berg K. A new serum type system in man--the LP system. Acta Pathol Microbiol Scand. 1963;59:369.
Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23.
Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–28.
• Emdin CA, Khera AV, Natarajan P, et al. Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol. 2016;68:2761–72 Results of this large phenome-wide association revealed that individuals with genetically lower Lp(a) levels have a substantially reduced risk of coronary artery disease, stroke, heart failure, peripheral artery disease, chronic kidney disease and calcific aortic valve stenosis.
Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–12.
Capoulade R, Chan KL, Yeang C, Mathieu P, Bossé Y, Dumesnil JG, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–46.
•• Wei JW-Q, Li LX, Feng WQ et al. LPA variants are associated with residual cardiovascular risk in patients receiving statins. Circulation 2018. Results of this large genome-wide association study revealed that a variant at the LPA locus was the only genome-wide significant single nucleotide polymorphism associated with residual risk of cardiovascular disease in patients treated with statins.
Berk K, Yahya R, Verhoeven A, et al. Effect of diet-induced weight loss on lipoprotein(a) levels in obese individuals with and without type 2 diabetes. Diabetologia. 2017;60:989–97.
Silaste LM-L, Rantala AM, Alfthan AG, et al. Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein(a). Arterioscler, Thromb Vasc Biol: J Am Heart Assoc. 2004;24:498–503.
Faghihnia N, Tsimikas S, Miller ER, Witztum JL, Krauss RM. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. 2010;51:3324–30.
Ginsberg HN, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, et al. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the Delta study, protocol 1. Arterioscler Thromb Vasc Biol. 1998;18:441–9.
Shin M-J, Blanche PJ, Rawlings RS, Fernstrom HS, Krauss RM. Increased plasma concentrations of lipoprotein(a) during a low-fat, high-carbohydrate diet are associated with increased plasma concentrations of apolipoprotein C-III bound to apolipoprotein B-containing lipoproteins.(original research communications). Am J Clin Nutr. 2007;85:1527–32.
Berglund L, Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, Stewart PW, et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states.(Author abstract). Am J Clin Nutr. 2007;86:1611–20.
Haring B, von Ballmoos MC, Appel LJ, Sacks FM. Healthy dietary interventions and lipoprotein (a) plasma levels: results from the Omni heart trial. PLoS One. 2014;9:e114859.
Najjar RS, Moore CE, Montgomery BD. Consumption of a defined, plant-based diet reduces lipoprotein(a), inflammation, and other atherogenic lipoproteins and particles within four weeks. Clin Cardiol 2018.
Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106:1327–32.
Jenkins DJ, Kendall CW, Marchie A, et al. The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism. 2003;52:1478–83.
Dodin S, Cunnane SC, Masse B, et al. Flaxseed on cardiovascular disease markers in healthy menopausal women: a randomized, double-blind, placebo-controlled trial. Nutrition. 2008;24:23–30.
Simental-Mendía LE, Gotto AM, Atkin SL, Banach M, Pirro M, Sahebkar A. Effect of soy isoflavone supplementation on plasma lipoprotein(a) concentrations: a meta-analysis. J Clin Lipidol. 2018;12:16–24.
Sahebkar A, Serban C, Ursoniu S, Banach M. Effect of garlic on plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of randomized controlled clinical trials. Nutrition. 2016;32:33–40.
Sahebkar A, Simental-Mendía LE, Stefanutti C, Pirro M. Supplementation with coenzyme Q10 reduces plasma lipoprotein(a) concentrations but not other lipid indices: a systematic review and meta-analysis. Pharmacol Res. 2016;105:198–209.
Maria-Corina S, Amirhossein S, Dimitri PM et al. Impact of L-carnitine on plasma lipoprotein(a) concentrations: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 2016;6.
Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–6.
Akesson A, Larsson SC, Discacciati A, Wolk A. Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol. 2014;64:1299–306.
Lachman S, Peters RJ, Lentjes MA, et al. Ideal cardiovascular health and risk of cardiovascular events in the EPIC-Norfolk prospective population study. Eur J Prev Cardiol. 2015.
•• Perrot N, Verbeek R, Sandhu M et al. Ideal cardiovascular health influences cardiovascular disease risk associated with high lipoprotein(a) levels and genotype: the EPIC-Norfolk prospective population study. Atherosclerosis 2017. This study performed in a large population-based prospective study showed that individuals characterized by “ideal cardiovascular health” may be at substantially reduced cardiovascular disease risk, even if they have elevated Lp(a) levels or if they carry the Lp(a)-raising variant rs10455872.
Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). Circulation. 2014;129:635–42.
Arsenault BJ, Barter P, DeMicco DA, et al. Prediction of cardiovascular events in statin-treated stable coronary patients of the treating to new targets randomized controlled trial by lipid and non-lipid biomarkers. PLoS One. 2014;9:e114519.
Arsenault BJ, Petrides F, Tabet F, Bao W, Hovingh GK, Boekholdt SM, et al. Effect of atorvastatin, cholesterol ester transfer protein inhibition, and diabetes mellitus on circulating proprotein subtilisin kexin type 9 and lipoprotein(a) levels in patients at high cardiovascular risk. J Clin Lipidol. 2018;12:130–6.
Yeang C, Hung M-Y, Byun Y-S, Clopton P, Yang X, Witztum JL, et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J Clin Lipidol. 2016;10:594–603.
Hopewell JC, Parish S, Offer A, Link E, Clarke R, Lathrop M, et al. Impact of common genetic variation on response to simvastatin therapy among 18 705 participants in the heart protection study. Eur Heart J. 2013;34:982–92.
Sahebkar A, Reiner Ž, Simental-Mendía LE, Ferretti G, Cicero AFG. Effect of extended-release niacin on plasma lipoprotein(a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism. 2016;65:1664–78.
Parish S, Hopewell JC, Hill MR, et al. Impact of apolipoprotein(a) isoform size on lipoprotein(a) lowering in the HPS2-THRIVE study. Circulation Genomic and precision medicine. 2018;11:e001696.
Croyal M, Ouguerram K, Passard M, Ferchaud-Roucher V, Chétiveaux M, Billon-Crossouard S, et al. Effects of extended-release nicotinic acid on apolipoprotein (a) kinetics in Hypertriglyceridemic patients. Arterioscler, Thromb, Vasc Biol. 2015;35:2042–7.
Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67.
Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12.
Shapiro MD, Tavori H, Fazio S. From basic science discoveries to clinical trials. Circ Res. 2018;122:1420–38.
Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–1272.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
• Verbeek R, Boyer M, Boekholdt SM, et al. Carriers of the PCSK9 R46L variant are characterized by an antiatherogenic lipoprotein profile assessed by nuclear magnetic resonance spectroscopy—brief report. Arterioscler Thromb Vasc Biol. 2017;37:43–8 Results of this study suggest that carriers of a variant associated with low proprotein convertase subtilisin/kexin type 9 may be characterized by lower levels of most atherogenic lipoprotein concentrations, including Lp(a).
Langsted GA, Nordestgaard RB, Benn RM, Tybjærg-Hansen RA, Kamstrup RP. PCSK9 R46L loss-of-function mutation reduces lipoprotein(a), LDL cholesterol, and risk of aortic valve stenosis. J Clin Endocrinol Metab. 2016;101:3281–7.
Desai NR, Kohli P, Giugliano RP, O'Donoghue ML, Somaratne R, Zhou J, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C assessment with proprotein convertase Subtilisin Kexin type 9 monoclonal antibody inhibition combined with statin therapy (LAPLACE)-thrombolysis in myocardial infarction (TIMI) 57 trial. Circulation. 2013;128:962–9.
Van Der Valk MF, Bekkering DS, Kroon JJ, et al. Oxidized phospholipids on lipoprotein(a) elicit Arterial Wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–24.
Reyes-Soffer MG, Pavlyha HM, Ngai RC, et al. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2017;135:352–62.
Watts GF, Chan DC, Somaratne R, Wasserman SM, Scott R, Marcovina SM, et al. Controlled study of the effect of proprotein convertase subtilisin-kexin type 9 inhibition with evolocumab on lipoprotein(a) particle kinetics. Eur Heart J. 2018;39:2577–85.
•• Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–53 This study shows that very important Lp(a) reduction could be obtained following administration of the antisense oligonucleotide targeting LPA AKCEA-APO(A)L RX.
•• Joshi P, Pirastu N, Kentistou K, et al. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nature Communications. 2017;8:910–0 Results of this study showed for the first time that variants at the LPA locus associated with low Lp(a) levels might be associated with human longevity.
Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53.
Acknowledgements
We would like to thank Dr. Patricia Mitchell for the helpful comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Audrey-Anne Després is supported by a master’s training award from the Fonds de recherche du Québec: Santé (FRQS) and Canadian Institutes of Health Research and declares no conflicts of interest. Benoit Arsenault holds a junior scholar award from the FRQS and has received research funding from the Canadian Institutes of Health Research (FRN155226 and FRN149068), Pfizer, Merck and Ionis Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Topical Collection on Lipids
Rights and permissions
About this article
Cite this article
Després, AA., Arsenault, B.J. Lipoprotein(a)—It Is Risky, but What Do We Do About It?. Curr Cardiovasc Risk Rep 12, 27 (2018). https://doi.org/10.1007/s12170-018-0592-7
Published:
DOI: https://doi.org/10.1007/s12170-018-0592-7