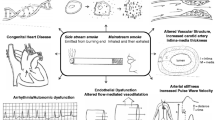
Abstract
Thanks to the foresight of researchers in the 1970s and 1980s in establishing large cohorts of children to investigate the origins of cardiovascular disease (CVD), we now have the ability to understand the impact childhood smoking exposure, among other pediatric exposures, has on later-life cardiovascular (CV) health. The age of the participants in these large prospective cohorts is still prohibitive in that researchers can only consider pre-clinical markers of CV pathology. Despite this, cohorts from multiple countries have reported consistent findings concerning the adverse CV impact childhood passive smoking exposure is having decades later. Fortunately, we now have sufficient evidence as to the exact mechanisms through which tobacco smoke causes CVD. With a large proportion of the world’s children exposed to passive smoking daily, especially in the as-yet unstudied developing world, but with very few living in jurisdictions with effective and enforced protective legislation, the time for public health action is now.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as • Of importance •• Of major importance
Schonherr E. Contribution to the statistical and clinical features of lung tumours (in German). Z Krebsforsch. 1928;27:436–50.
English JP, Willius FA, Berkson J. Tobacco and coronary disease. JAMA. 1940;115:1327–9.
Smoking NRCUCoP. Environmental tobacco smoke: measuring exposures and assessing health effects (1986). 2015.
Lightwood JM, Glantz SA. Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation. 2009;120:1373–9.
Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012;126:2177–83. A meta-analysis of 45 studies revealed comprehensive smoke-free legislation was associated with significantly lower rates of hospital admissions and deaths for coronary events, cerebrovascular accidents, respiratory disease and other heart disease. The difference in risk following comprehensive smoke-free laws does not change with longer follow-up and more comprehensive laws were associated with larger changes in risk.
Glantz SA, Parmley WW. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation. 1991;83:1–12.
Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, adult risk factors and incident coronary heart disease: the Caerphilly Study. Pub Health. 1996;110:139–43.
Magnussen CG, Smith KJ, Juonala M. What the long term cohort studies that began in childhood have taught us about the origins of coronary heart disease. Curr Cardiovasc Risk Rep. 2014;8:373.
Gall S, Huynh QL, Magnussen CG, Juonala M, Viikari JS, Kahonen M, et al. Exposure to parental smoking in childhood or adolescence is associated with increased carotid intima-media thickness in young adults: evidence from the cardiovascular risk in young Finns study and the childhood determinants of adult health study. Eur Heart J. 2014;35:2484–91. Children exposed to parental smoking, confirmed by serum cotinine, were significantly more likely to have carotid plaque in adulthood as opposed to those not exposed. Children of parents that maintained good smoking hygiene, by smoking in a way that did not expose their children, were significantly less likely to have developed carotid artery plaque in adulthood.
Geerts CC, Bots ML, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in young adult offspring: is early life exposure critical? The atherosclerosis risk in young adults study. Arterioscler Thromb Vasc Biol. 2008;28:2296–302.
West HW, Juonala M, Gall SL, Kahonen M, Laitinen T, Taittonen L, et al. Exposure to parental smoking in childhood is associated with increased risk of carotid atherosclerotic plaque in adulthood: the cardiovascular risk in young Finns study. Circulation. 2015;131:1239–46. Children exposed to parental smoking, confirmed by serum cotinine, were significantly more likely to have carotid plaque in adulthood as opposed to those not exposed. Children of parents that maintained good smoking hygiene, by smoking in a way that did not expose their children, were significantly less likely to have developed carotid artery plaque in adulthood.
Juonala M, Magnussen CG, Venn A, Gall S, Kähönen M, Laitinen T, et al. Parental smoking in childhood and brachial artery flow-mediated dilatation in young adults: the cardiovascular risk in young Finns study and the childhood determinants of adult health study. Arterioscler Thromb Vasc Biol. 2012;32:1024–31. Evidence from two major longitudinal cohorts that children exposed to parental smoking in childhood have significantly attenuated brachial artery flow-mediated dilatation, an important marker of endothelial function, in adulthood.
Kvalvik LG, Nilsen RM, Skjaerven R, Vollset SE, Midttun O, Ueland PM, et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr Res. 2012;72:101–7.
Perez-Rios M, Schiaffino A, Lopez MJ, Nebot M, Galan I, Fu M, et al. Questionnaire-based second-hand smoke assessment in adults. Eur J Pub Health. 2013;23:763–7.
Gaffney KF, Molloy SB, Maradiegue AH. Questionnaires for the measurement of infant environmental tobacco smoke exposure: a systematic review. J Nurs Meas. 2003;11:225–39.
Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107 Suppl 2:349–55.
Jaakkola MS, Jaakkola JJ. Assessment of exposure to environmental tobacco smoke. Eur Respir J. 1997;10:2384–97.
Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23:47–53.
Kallio K, Jokinen E, Hamalainen M, Kaitosaari T, Volanen I, Viikari J, et al. Impact of repeated lifestyle counselling in an atherosclerosis prevention trial on parental smoking and children’s exposure to tobacco smoke. Acta Paediatr. 2006;95:283–90.
Matt GE, Quintana PJ, Zakarian JM, Fortmann AL, Chatfield DA, Hoh E, et al. When smokers move out and non-smokers move in: residential thirdhand smoke pollution and exposure. Tob Control. 2011;20:e1.
Kuschner WG, Reddy S, Mehrotra N, Paintal HS. Electronic cigarettes and thirdhand tobacco smoke: two emerging health care challenges for the primary care provider. Int J Gen Med. 2011;4:115–20.
Ferrante G, Simoni M, Cibella F, Ferrara F, Liotta G, Malizia V, et al. Third-hand smoke exposure and health hazards in children. Monaldi Arch Chest Dis. 2013;79:38–43.
Glantz SA, Parmley WW. Passive smoking and heart disease. Mechanisms and risk. JAMA. 1995;273:1047–53.
Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–98.
Juonala M, Magnussen CG, Raitakari OT. Parental smoking produces long-term damage to vascular function in their children. Curr Opin Cardiol. 2013;28:569–74.
Moffatt RJ, Stamford BA, Biggerstaff KD. Influence of worksite environmental tobacco smoke on serum lipoprotein profiles of female nonsmokers. Metab Clin Exp. 1995;44:1536–9.
Moffatt RJ, Chelland SA, Pecott DL, Stamford BA. Acute exposure to environmental tobacco smoke reduces HDL-C and HDL2-C. Prev Med. 2004;38:637–41.
Mizoue T, Ueda R, Hino Y, Yoshimura T. Workplace exposure to environmental tobacco smoke and high density lipoprotein cholesterol among nonsmokers. Am J Epidemiol. 1999;150:1068–72.
Jaddoe VW, de Ridder MA, van den Elzen AP, Hofman A, Uiterwaal CS, Witteman JC. Maternal smoking in pregnancy is associated with cholesterol development in the offspring: a 27-years follow-up study. Atherosclerosis. 2008;196:42–8.
Ayer JG, Belousova E, Harmer JA, David C, Marks GB, Celermajer DS. Maternal cigarette smoking is associated with reduced high-density lipoprotein cholesterol in healthy 8-year-old children. Eur Heart J. 2011;32:2446–53.
Moriguchi EH, Fusegawa Y, Tamachi H, Goto Y. Effects of smoking on HDL subfractions in myocardial infarction patients: effects on lecithin-cholesterol acyltransferase and hepatic lipase. Clin Chim Acta. 1991;195:139–43.
Valkonen M, Kuusi T. Passive smoking induces atherogenic changes in low-density lipoprotein. Circulation. 1998;97:2012–6.
Davis JW, Shelton L, Watanabe IS, Arnold J. Passive smoking affects endothelium and platelets. Arch Intern Med. 1989;149:386–9.
Burghuber OC, Punzengruber C, Sinzinger H, Haber P, Silberbauer K. Platelet sensitivity to prostacyclin in smokers and non-smokers. Chest. 1986;90:34–8.
Lassila R, Laustiola KE. Cigarette smoking and platelet-vessel wall interactions. Prostaglandins Leukot Essent Fat Acids. 1992;46:81–6.
Shima M, Adachi M. Effects of environmental tobacco smoke on serum levels of acute phase proteins in schoolchildren. Prev Med. 1996;25:617–24.
Wilkinson JD, Lee DJ, Arheart KL. Secondhand smoke exposure and C-reactive protein levels in youth. Nicotine Tob Res. 2007;9:305–7.
Nakata A, Tanigawa T, Araki S, Sakurai S, Iso H. Lymphocyte subpopulations among passive smokers. JAMA. 2004;291:1699–700.
Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Masoura C, Toutouzas P, et al. Effect of exposure to secondhand smoke on markers of inflammation: the ATTICA study. Am J Med. 2004;116:145–50.
Sobczak A, Wardas W, Zielinska-Danch W, Pawlicki K. The influence of smoking on plasma homocysteine and cysteine levels in passive and active smokers. Clin Chem Lab Med CCLM FESCC. 2004;42:408–14.
Diez-Roux AV, Nieto FJ, Comstock GW, Howard G, Szklo M. The relationship of active and passive smoking to carotid atherosclerosis 12–14 years later. Prev Med. 1995;24:48–55.
Cucina A, Sapienza P, Borrelli V, Corvino V, Foresi G, Randone B, et al. Nicotine reorganizes cytoskeleton of vascular endothelial cell through platelet-derived growth factor BB. J Surg Res. 2000;92:233–8.
Stefanadis C, Vlachopoulos C, Tsiamis E, Diamantopoulos L, Toutouzas K, Giatrakos N, et al. Unfavorable effects of passive smoking on aortic function in men. Ann Intern Med. 1998;128:426–34.
Mack WJ, Islam T, Lee Z, Selzer RH, Hodis HN. Environmental tobacco smoke and carotid arterial stiffness. Prev Med. 2003;37:148–54.
Mahmud A, Feely J. Effects of passive smoking on blood pressure and aortic pressure waveform in healthy young adults—influence of gender. Br J Clin Pharmacol. 2004;57:37–43.
Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–7.
Otsuka R, Watanabe H, Hirata K, Tokai K, Muro T, Yoshiyama M, et al. Acute effects of passive smoking on the coronary circulation in healthy young adults. JAMA. 2001;286:436–41.
Kato M, Roberts-Thomson P, Phillips BG, Narkiewicz K, Haynes WG, Pesek CA, et al. The effects of short-term passive smoke exposure on endothelium-dependent and independent vasodilation. J Hypertens. 1999;17:1395–401.
Lekakis J, Papamichael C, Vemmos C, Stamatelopoulos K, Voutsas A, Stamatelopoulos S. Effects of acute cigarette smoking on endothelium-dependent arterial dilatation in normal subjects. Am J Cardiol. 1998;81:1225–8.
Woo KS, Chook P, Leong HC, Huang XS, Celermajer DS. The impact of heavy passive smoking on arterial endothelial function in modernized Chinese. J Am Coll Cardiol. 2000;36:1228–32.
Sumida H, Watanabe H, Kugiyama K, Ohgushi M, Matsumura T, Yasue H. Does passive smoking impair endothelium-dependent coronary artery dilation in women? J Am Coll Cardiol. 1998;31:811–5.
Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–4.
Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Celermajer DS. Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Ann Intern Med. 1999;130:578–81.
Schwandt P, Haas GM, Liepold E. Lifestyle and cardiovascular risk factors in 2001 child–parent pairs: the PEP Family Heart Study. Atherosclerosis. 2010;213:642–8.
Weitzman M, Cook S, Auinger P, Florin TA, Daniels S, Nguyen M, et al. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005;112:862–9.
Khanolkar AR, Byberg L, Koupil I. Parental influences on cardiovascular risk factors in Swedish children aged 5–14 years. Eur J Pub Health. 2012;22:840–7.
Kallio K, Jokinen E, Saarinen M, Hämäläinen M, Volanen I, Kaitosaari T, et al. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circ Cardiovasc Qual Outcomes. 2010;3:196–203.
Moskowitz WB, Mosteller M, Schieken RM, Bossano R, Hewitt JK, Bodurtha JN, et al. Lipoprotein and oxygen transport alterations in passive smoking preadolescent children. The MCV Twin Study. Circulation. 1990;81:586–92.
Neufeld EJ, Mietus-Snyder M, Beiser AS, Baker AL, Newburger JW. Passive cigarette smoking and reduced HDL cholesterol levels in children with high-risk lipid profiles. Circulation. 1997;96:1403–7.
Feldman J, Shenker IR, Etzel RA, Spierto FW, Lilienfield DE, Nussbaum M, et al. Passive smoking alters lipid profiles in adolescents. Pediatrics. 1991;88:259–64.
McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang CC, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ Health Perspect. 2014;123:360–6.
Prevention CfDCa. Current cigarette smoking among adults—United States, 2005–2013. Morb Mortal Wkly Rep. 2014;63:1108–12.
Kallio K, Jokinen E, Hamalainen M, Saarinen M, Volanen I, Kaitosaari T, et al. Decreased aortic elasticity in healthy 11-year-old children exposed to tobacco smoke. Pediatrics. 2009;123:e267–73.
Geerts CC, Bots ML, van der Ent CK, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in their 5-year-old children. Pediatrics. 2012;129:45–54. Children exposed to maternal smoking in-utero had both significantly thicker carotid artery IMT and lower distensibility at age 5 years. The association did not hold for mothers who only smoked after pregnancy, perhaps do to the short follow-up in comparison to other cohorts.
Kallio K, Jokinen E, Raitakari OT, Hamalainen M, Siltala M, Volanen I, et al. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115:3205–12.
Laitinen TT, Pahkala K, Venn A, Woo JG, Oikonen M, Dwyer T, et al. Childhood lifestyle and clinical determinants of adult ideal cardiovascular health: the cardiovascular risk in Young Finns Study, the childhood Determinants of adult health study, the Princeton Follow-Up Study. Int J Cardiol. 2013;169:126–32. Findings from three major cohorts located on different continents that amongst other lifestyle and clinical indicators, higher family socioeconomic status and non-smoking (parental/own) in childhood independently predict ideal cardiovascular health in adulthood, as defined by the American Heart Association.
Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–6.
Pulkki-Raback L, Elovainio M, Hakulinen C, Lipsanen J, Hintsanen M, Jokela M, et al. Cumulative effect of psychosocial factors in youth on ideal cardiovascular health in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2015;131:245–53.
Chen W, Yun M, Fernandez C, Li S, Sun D, Lai CC, et al. Secondhand smoke exposure is associated with increased carotid artery intima-media thickness: the Bogalusa Heart Study. Atherosclerosis. 2015;240:374–9. Exposure to second-hand-smoke in childhood, as measured by retrospective questionnaire, was significantly associated with adulthood carotid artery IMT and showed a relatively stronger association with increased carotid IMT than the exposure in adulthood.
Laitinen TT, Pahkala K, Magnussen CG, Viikari JS, Oikonen M, Taittonen L, et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2012;125:1971–8.
Stein JH, Fraizer MC, Aeschlimann SE, Nelson-Worel J, McBride PE, Douglas PS. Vascular age: integrating carotid intima-media thickness measurements with global coronary risk assessment. Clin Cardiol. 2004;27:388–92.
Wannarong T, Parraga G, Buchanan D, Fenster A, House AA, Hackam DG, et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke. 2013;44:1859–65.
Mendelson MM, de Ferranti SD. Childhood environmental tobacco smoke exposure: a smoking gun for atherosclerosis in adulthood. Circulation. 2015;131:1231–3.
Ho SY, Wang MP, Lo WS, Mak KK, Lai HK, Thomas GN, et al. Comprehensive smoke-free legislation and displacement of smoking into the homes of young children in Hong Kong. Tob Control. 2010;19:129–33.
Picard A. Parents abuse children by smoking, group says. Globe Mail. 2003. A meta-analysis of 45 studies revealed comprehensive smoke-free legislation was associated with significantly lower rates of hospital admissions and deaths for coronary events, cerebrovascular accidents, respiratory disease, and other heart disease. The difference in risk following comprehensive smoke-free laws does not change with longer follow-up, and more comprehensive laws were associated with larger changes in risk
From the American Academy of Pediatrics. Policy statement—tobacco use: a pediatric disease. Pediatrics. 2009;124:1474–87.
Jones MR, Barnoya J, Stranges S, Losonczy L, Navas-Acien A. Cardiovascular events following smoke-free legislations: an updated systematic review and meta-analysis. Curr Environ Health Rep. 2014;1:239–49.
Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–46.
World Health Organization. Global health risks: mortality and burden of diseases attributable to selected major risks. Geneva: World Health Organization; 2009.
Veeranki SP, Mamudu HM, Zheng S, John RM, Cao Y, Kioko D, et al. Secondhand smoke exposure among never-smoking youth in 168 countries. J Adolesc Health. 2015;56:167–73. Important data obtained via the Global Youth Tobacco Surveys conducted in 168 countries during 1999-2008. Of 356,414 never-smoking adolescents included in the study, 30.4 %, 44.2 %, and 23.2 % were exposed to SHS inside home, outside home, and both, respectively.
WHO. Report on the global tobacco epidemic 2008: The MPOWER package. Geneva: World Health Organization; 2008.
Acknowledgments
We acknowledge the participants in the youth-to-adult cohorts from around the world who have enabled our knowledge of CVD risk factors to develop for the good of others. We also acknowledge the Chief Investigators of the studies highlighted in this review for their forethought and invaluable contributions to the field.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Henry West reports grants from The Australian National Heart Foundation, during the conduct of the study. Costan Magnussen, Markus Juonala, and Seana Gall have no relevant disclosures to report.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pediatrics
Rights and permissions
About this article
Cite this article
West, H.W., Gall, S.L., Juonala, M. et al. Is Passive Smoking Exposure in Early Life a Risk Factor for Future Cardiovascular Disease?. Curr Cardiovasc Risk Rep 9, 42 (2015). https://doi.org/10.1007/s12170-015-0471-4
Published:
DOI: https://doi.org/10.1007/s12170-015-0471-4