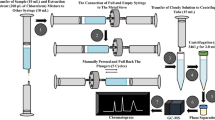
Abstract
Pigeon is an essential poultry with tasty meat and high protein content, and its breeding and consumption of meat have been increasing annually. Nevertheless, technical bottlenecks still exist in detecting veterinary drug residues in pigeon muscle. A rapid and sensitive method for determining 67 veterinary drugs belonging to six major groups (quinolones, sulfonamides, macrolides, hormones, tetracyclines, and insecticides) was developed in pigeon muscle. The method described a QuEChERS sample preparation procedure before liquid chromatography−tandem mass spectrometry (LC-MS/MS). The modified QuEChERS extraction method was optimized using the cost-effective chemometric tool equipped with Plackett-Burman (P-B) and Box-Behnken (BBD) response surface experiment designs. Based on the response surface methodology, the optimized conditions were 85.0% methanol, 408.1 mg C18, and 108.3 mg Z-Sep+. LC-MS/MS performed the quantitative detection with multiple reaction monitoring (MRM) modes under positive ion electrospray ionization (ESI+). The calibration curves showed good linearity in the corresponding concentration ranges, with the coefficients of determination (R2) all greater than 0.99. The method’s linearity, selectivity, intra-day and inter-day precision, accuracy, decision limit, detection capability, uncertainty, and quantitation limits are reported. The resulting limits of quantitation were 0.5–2.0 μg kg−1, and therefore satisfactory. Our method was applied to actual samples and proved adequate for routine analysis. The proposed method proved simple, rapid, and reliable for monitoring 67 veterinary drugs in pigeon muscle and can also be used for food safety monitoring.






Similar content being viewed by others
Data Availability
The data analyzed during the current study are available from the corresponding author on reasonable request.
References
[EC] European Commission (2010) Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union L 15:1–76
[EC] European Commission (2021) Commission implementing regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing Decisions 2002/657/EC and 98/179/EC. Off J Eur Union L 180:84–109 Available online: http://data.europa.eu/eli/reg_impl/2021/808/oj Accessed 22 Jan 2024
Abdel-Moety EM, Rezk MR, Wadie MT, Tantawy MA (2021) A combined approach of green chemistry and quality-by-design for sustainable and robust analysis of two newly introduced pharmaceutical formulations treating benign prostate hyperplasia. Microchem J 160:105711. https://doi.org/10.1016/j.microc.2020.105711
Abdulra'uf LB, Sirhan AY, Tan GH (2016) Applications of experimental design to the optimization of microextraction sample preparation parameters for the analysis of pesticide residues in fruits and vegetables. J AOAC Int 99(6):1415–1425. https://doi.org/10.5740/jaoacint.sge3abdulrauf
Abdulra'uf LB, Tan GH (2015) Chemometric approach to the optimization of HS-SPME/GC-MS for the determination of multiclass pesticide residues in fruits and vegetables. Food Chem 177:267–273. https://doi.org/10.1016/j.foodchem.2015.01.031
Bărbulescu A, Barbeș L (2022) Challenges and opportunities in the application of chemometrics in the pharmaceutical and food science industries. J Chem 9823497. https://doi.org/10.1155/2022/9823497
Bezerra MA, Ferreira SLC, Novaes CG, Dos Santos AMP, Valasques GS, da Mata Cerqueira UMF, dos Santos Alves JP (2019) Simultaneous optimization of multiple responses and its application in analytical chemistry-a review. Talanta 194:941–959. https://doi.org/10.1016/j.talanta.2018.10.088
Chang L-L, Tang Q-P, Zhang R, Fu S-Y, Mu C-Y, Shen X-Y, Bu Z (2023) Evaluation of meat quality of local pigeon varieties in China. Animals 13(8):1291. https://doi.org/10.3390/ani13081291
Chen J, Ying G-G, Deng W-J (2019a) Antibiotic residues in food: extraction, analysis, and human health concerns. J Agric Food Chem 67(27):7569–7586. https://doi.org/10.1021/acs.jafc.9b01334
Chen Q, Pan XD, Huang BF, Han JL (2017) Quantification of 16 β-lactams in chicken muscle by QuEChERS extraction and UPLC-Q-Orbitrap-MS with parallel reaction monitoring. J Pharmaceut Biomed 145(3399):525–530. https://doi.org/10.1016/j.jpba.2017.07.019
Chen X-L, Ai L-F, Cao Y-Q, Nian Q-X, Jia Y-Q, Hao Y-L, Wang M-M, Wang X-S (2019b) Rapid determination of sulfonamides in chicken muscle and milk using efficient graphene oxide based monolith on-line solid-phase extraction coupled with liquid chromatography–tandem mass spectrometry. Food Anal Methods 12(1):271–281. https://doi.org/10.1007/s12161-018-1358-z
Chitescu CL, Nicolau AI, Csuma A, Moisoiu C (2011) Simultaneous analysis of four sulfonamides in chicken muscle tissue by HPLC. Food Addit Contam A 28(8):1013–1020. https://doi.org/10.1080/19440049.2011.577098
Danezis GP, Anagnostopoulos CJ, Liapis K, Koupparis MA (2016) Multi-residue analysis of pesticides, plant hormones, veterinary drugs and mycotoxins using HILIC chromatography-MS/MS in various food matrices. Anal Chim Acta 942:121–138. https://doi.org/10.1016/j.aca.2016.09.011
Delatour T, Racault L, Bessaire T, Desmarchelier A (2018) Screening of veterinary drug residues in food by LC-MS/MS. Background and challenges. Food Addit Contam A 35(4):633–646. https://doi.org/10.1080/19440049.2018.1426890
Desmarchelier A, Bessaire T, Savoy MC, Tarres A, Mujahid C, Beck A, Mottier P, Delatour T (2021) Screening of 154 veterinary drug residues in foods of animal origin using LC–MS/MS, first action 2020.04. J AOAC Int 104(3):650–681. https://doi.org/10.1093/jaoacint/qsaa168
Ellison SLR and Williams A (Eds). Eurachem/CITAC guide: quantifying uncertainty in analytical measurement, 3rd edn., (EURACHEM/CITAC, Europe, 2012) https://www.eurachem.org
Fayissa GR, Dube S, Nindi MM (2023) Fast screening method to determine metabolites of nitrofurans in chicken meat using partitioned dispersive liquid–liquid microextraction combined with HPLC/DAD. Food Addit Contam A 40(1):56–66. https://doi.org/10.1080/19440049.2022.2136767
Ferreira SLC, Lemos VA, de Carvalho VS, da Silva EGP, Queiroz AFS, Felix CSA, da Silva DLF, Dourado GB, Oliveira RV (2018) Multivariate optimization techniques in analytical chemistry-an overview. Microchem J 140:176–182. https://doi.org/10.1016/j.microc.2018.04.002
Garcia CV, Gotah A (2017) Application of QuEChERS for determining xenobiotics in foods of animal origin. J Anal Methods Chem 2603067. https://doi.org/10.1155/2017/2603067
Gržinić G, Piotrowicz-Cieślak A, Klimkowicz-Pawlas A, Górny RL, Ławniczek-Wałczyk A, Piechowicz L, Olkowska E, Potrykus M, Tankiewicz M, Krupka M, Siebielec G, Wolska L (2023) Intensive poultry farming: a review of the impact on the environment and human health. Sci Total Environ 858:160014. https://doi.org/10.1016/j.scitotenv.2022.160014
Gu H, Dai Y-Y, Li J, Xu S-J, Pan Z-X (2023) Factors affecting the quality and safety of pigeon products and countermeasures. China Anim Ind 02:70–71 https://www.cnki.net
Han Z, Zhang L, Huang Y (2016) China's meat pigeon breeding status and prospects for benefit analysis. Henan J Anim Hus Vet Med 37(6):22–23. https://doi.org/10.3969/j.issn.1004-5090.2016.06.010
Jammoul A, Darra NE (2019) Evaluation of antibiotics residues in chicken meat samples in Lebanon. Antibiotics 8(2):1–11. https://doi.org/10.3390/antibiotics8020069
Jongedijk E, Fifeik M, Arrizabalaga-Larrañaga A, Polzer J, Blokland M, Sterk S (2023) Use of high-resolution mass spectrometry for veterinary drug multi-residue analysis. Food Control 145:109488. https://doi.org/10.1016/j.foodcont.2022.109488
Kaufmann A, Butcher P, Maden K, Walker S, Widmer M (2023) Assessment and validation of the p-QuEChERS sample preparation methodology for the analysis of > 200 veterinary drugs in various animal-based food matrices. Food Addit Contam A 40(3):356–372. https://doi.org/10.1080/19440049.2023.2171142
Khodadoust S, Ghaedi M, Hadjmohammadi MR (2013) Dispersive nano solid material-ultrasound assisted microextraction as a novel method for extraction and determination of bendiocarb and promecarb: Response surface methodology. Talanta 116:637–646. https://doi.org/10.1016/j.talanta.2013.07.013
Kowalski P, Plenis A, Oledzka I, Konieczna L (2011) Optimization and validation of the micellar electrokinetic capillary chromatographic method for simultaneous determination of sulfonamide and amphenicol-type drugs in poultry tissue. J Pharmaceut Biomed 54:160–167. https://doi.org/10.1016/j.jpba.2010.08.005
Ministry of Agriculture and Rural Affairs of the People’s Republic of China (2020) National Food Safety Standard-maximum residue limits for veterinary drugs in foods. GB31650–2019. Standards Press of China: Beijing, China, 2020. Available online: http://31650.foodmate.net/. Accessed 19 Dec 2023
Moga A, Vergara-Barberán M, Lerma-García MJ, Carrasco-Correa EJ, Herrero-Martínez JM, Simó-Alfonso EF (2021) Determination of antibiotics in meat samples using analytical methodologies: A review. Compr Rev Food Sci F 20(2):1681–1716. https://doi.org/10.1111/1541-4337.12702
Moretti S, Dusi G, Giusepponi D, Pellicciotti S, Rossi R, Saluti G, Cruciani G, Galarini R (2016) Screening and confirmatory method for multiclass determination of 62 antibiotics in meat. J Chromatogr A 1429:175–188. https://doi.org/10.1016/j.chroma.2015.12.021
Patel T, Marmulak T, Gehring R, Pitesky M, Clapham MO, Tell LA (2018) Drug residues in poultry meat: a literature review of commonly used veterinary antibacterials and anthelmintics used in poultry. J Vet Pharmacol Ther 41(6):761–789. https://doi.org/10.1111/jvp.12700
Peris-Vicente J, Peris-García E, Albiol-Chiva J, Durgbanshi A, Ochoa-Aranda E, Carda-Broch S, Bose D, Esteve-Romero J (2022) Liquid chromatography, a valuable tool in the determination of antibiotics in biological, food and environmental samples. Microchem J 177:107309. https://doi.org/10.1016/j.microc.2022.107309
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33(4):305–325. https://doi.org/10.1093/biomet/33.4.305
Pratiwi R, Ramadhanti SP, Amatulloh A, Megantara S, Subra L (2023) Recent advances in the determination of veterinary drug residues in food. Foods 12(18):3422. https://doi.org/10.3390/foods12183422
Tao Y-F, Chen D-M, Yu H, Huang L-L, Liu Z-Y, Cao X-Q, Yan C-X, Pan Y-H, Liu Z-L, Yuan Z-H (2012) Simultaneous determination of 15 aminoglycoside(s) residues in animal derived foods by automated solid-phase extraction and liquid chromatography-tandem mass spectrometry. Food Chem 135:676–683. https://doi.org/10.1016/j.foodchem.2012.04.086
Wang B, Xie K-Z, Lee K (2021) Veterinary drug residues in animal-derived foods: Sample preparation and analytical methods. Foods 10(3):555. https://doi.org/10.3390/foods10030555
Wang W, Li S-Q, Yin P-Y, Li J-X, Tang Y, Yang M (2022) Response surface methodology optimization for the synthesis of N, S-codoped carbon dots and its application for tetracyclines detection. Chemosphere. 303(2):135145. https://doi.org/10.1016/j.chemosphere.2022.135145
Yin Z-Q, Song Y, Chai T-T, Wang X-L, Jia Q, Yang S-M, Qiu J (2017) Recent development in multi-residue determination of drugs in animal derived food by ultra-high-performance liquid chromatography-tandem mass spectrometry research. Food Fermentation Ind 43(4):286–294. https://doi.org/10.13995/j.cnki.11-1802/ts.201704045
Zhang C-Y, Deng Y-C, Zheng J-F, Zhang Y, Yang L-H, Liao C-J, Su L, Zhou Y-Y, Gong D-X, Chen L, Luo A (2019) The application of the QuEChERS methodology in the determination of antibiotics in food: A review. TrAC-Trend Anal Chem 118:517–537. https://doi.org/10.1016/j.trac.2019.06.012
Zhang Y-X, Xue X, Su S-F, Guo Z-M, Wang J, Ding L-S, Liu Y-M, Zhu J-H (2018) A multi-class, multi-residue method for detection of veterinary drugs in multiple meat using a pass-through cleanup SPE technique and UPLC-MS/MS analysis. Food Anal Methods 11(10):2865–2884. https://doi.org/10.1007/s12161-018-1244-8
Zheng W-J, Abd El-Aty AM, Kim SK, Choi JM, Park DH, Yoo KH, Kang YS, Jeon JS, Hacımüftüoğlu A, Shim JH, Shin HC (2019) Development and validation of a solid-phase extraction method coupled with LC–MS/MS for the simultaneous determination of 16 antibiotic residues in duck meat. Biomed Chromatogr 33(5):1–10. https://doi.org/10.1002/bmc.4501
Acknowledgements
The authors acknowledge support from Waters Technology (Shanghai) Co., Ltd.
Funding
This work was supported by the fundamental research funds for the public research institutes of Chinese academy of inspection and quarantine under Grant 2020JK010.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Xingqiang Wu and Xiaoxuan Yu performed material preparation, data collection, and analysis. Xingqiang Wu wrote the main manuscript text. Kaixuan Tong and Yujie Xie prepared Figures 1–6 and Tables 1–2. Qiaoying Chang and Hui Chen rewrote the first draft of the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Ethical approval was not required for the study because the sampling process did not harm the animals.
Consent to Participate
Not applicable.
Conflict of Interest
Xingqiang Wu declares no conflict of interest. Xiaoxuan Yu declares no conflict of interest. Kaixuan Tong declares no conflict of interest. Yujie Xie declares no conflict of interest. Qiaoying Chang declares no conflict of interest. Chunlin Fan declares no conflict of interest. Minglin Wang declares no conflict of interest. Hui Chen declares no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary file 1
Fig. S1. Recovery rate and precision of 67 veterinary drugs in pigeon at three levels (5、10, and 50 μg kg−1). Table S1. Mass spectrum parameters of 67 veterinary drugs. Table S2. Plackett-Burman experimental design table and results. Table S3. Variance analysis of regression model of veterinary drugs extraction from spiked samples. Table S4. Design and results of response surface experiment. Table S5. Variance analysis of regression model of veterinary drugs extraction from spiked samples.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, X., Yu, X., Tong, K. et al. Chemometric−Assisted QuEChERS Method for Multiresidue Analysis of Veterinary Drugs in Pigeon Muscle by LC-MS/MS. Food Anal. Methods 17, 551–563 (2024). https://doi.org/10.1007/s12161-024-02589-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-024-02589-7