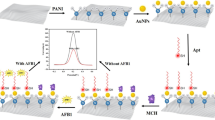
Abstract
Aflatoxin B1 (AFB1) is an important indicator in food safety assessment. In the present study, a non-label electrochemical aptasensor based on the Cu metal–organic framework (CuMOF) was fabricated to measure AFB1. The CuMOF was synthesized with a simple method and used to modify glassy carbon electrode (GCE). The field emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR) methods were used to describe the morphology, structure, and size of the CuMOF. It is established that the CuMOF with a large surface area played an important role as a unique substrate to immobilize AFB1 aptamer. The fabricating stages of the aptasensor were followed by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) using ferri/ferrocyanide as a redox probe. The results indicate that under optimal conditions, the aptamer-AFB1 interaction increases the resistance to electron transfer between them. Through an EIS method, the linear range of 1.0 × 10−3 to 200.0 ng/mL and detection limit of 8.3 × 10−4 ng/mL were obtained for quantitative determination of AFB1 at the aptasensor surface. In addition, the proposed aptasensor has acceptable reproducibility, repeatability, and stability to determine AFB1. Finally, this method has been successfully used for the analysis of AFB1 in wheat flour samples.






Similar content being viewed by others
References
Acquah C, Danquah MK, Yon JL, Sidhu A, Ongkudon CM (2015) A review on immobilised aptamers for high throughput biomolecular detection and screening. Anal Chim Acta 888:10–18
Adachi T, Nakamura Y (2019) Aptamers: a review of their chemical properties and modifications for therapeutic application. Molecules 24:4229
Cavaliere C, Foglia P, Guarino C, Nazzari M, Samperi R, Laganà A (2007) Determination of aflatoxins in olive oil by liquid chromatography–tandem mass spectrometry. Anal Chim Acta 596:141–148
Chen L, Wen F, Li M, Guo X, Li S, Zheng N, Wang J (2017) A simple aptamer-based fluorescent assay for the detection of aflatoxin B1 in infant rice cereal. Food Chem 215:377–382
Chen Q, Yang M, Yang X, Li H, Guo Z, Rahma M (2018) A large Raman scattering cross-section molecular embedded SERS aptasensor for ultrasensitive aflatoxin B1 detection using CS-Fe3O4 for signal enrichment. Spectrochim Acta A 189:147–153
Costa MP, Frías IA, Andrade CA, Oliveira MD (2017) Impedimetric immunoassay for aflatoxin B1 using a cysteine modified gold electrode with covalently immobilized carbon nanotubes. Microchim Acta 184:3205–3213
Cui J, Feng Y, Lin T, Tan Z, Zhong C, Jia S (2017) Mesoporous metal–organic framework with well-defined cruciate flower-like morphology for enzyme immobilization. ACS Appl Mater Interfaces 9:10587–10594
Dridi F, Marrakchi M, Gargouri M, Saulnier J, Jaffrezic-Renault N, Lagarde F (2017) Nanomaterial-based electrochemical biosensors for food safety and quality assessment. Nanobiosensors 167–204
Evtugyn G, Porfireva A, Stepanova V, Sitdikov R, Stoikov I, Nikolelis D, Hianik T (2014) Electrochemical aptasensor based on polycarboxylic macrocycle modified with neutral red for aflatoxin B1 detection. Electroanalysis 26:2100–2109
Gathumbi JK, Usleber E, Ngatia TA, Kangethe EK, Martlbauer E (2003) Application of immunoaffinity chromatography and enzyme immunoassay in rapid detection of aflatoxin B1 in chicken liver tissues. Poult Sci J 82:585–590
Goud KY, Catanante G, Hayat A, Satyanarayana M, Gobi KV, Marty JL (2016) Disposable and portable electrochemical aptasensor for label free detection of aflatoxin B1 in alcoholic beverages. Sensor Actuat B Chem 235:466–473
Goud KY, Hayat A, Catanante G, Satyanarayana M, Gobi KV, Marty JL (2017) An electrochemical aptasensor based on functionalized graphene oxide assisted electrocatalytic signal amplification of methylene blue for aflatoxin B1 detection. Electrochim Acta 244:96–103
Grunker R, Bon V, Heerwig A, Klein N, Muller P, Stoeck U, Baburin IA, Mueller U, Senkovska I, Kaskel S (2012) Dye encapsulation inside a new mesoporous metal–organic framework for multifunctional solvatochromic-response function. Chem Eur J 18:13299–13303
Hu M, Zhang K (2013) The application of aptamers in cancer research: an up-to-date review. Future Oncol 9:369–376
Jahangiri–Dehaghani F, Zare HR, Shekari Z (2020) Measurement of aflatoxin M1 in powder and pasteurized milk samples by using a label–free electrochemical aptasensor based on platinum nanoparticles loaded on Fe–based metal–organic frameworks. Food Chem 310:125820
Jahangiri-Dehaghani F, Zare HR, Shekari Z (2021) Encapsulation of hemin in Fe-based metal-organic frameworks and its application for the direct determination of aflatoxin M1. World Mycotoxin J 1–10
Kotinagu K, Mohanamba T, Kumari LR (2015) Assessment of aflatoxin B1 in livestock feed and feed ingredients by high-performance thin layer chromatography. Vet World 8:1396
Leclerc H, Vimont A, Lavalley JC, Daturi M, Wiersum AD, Llwellyn PL, Horcajada P, Ferey G, Serre C (2011) Infrared study of the influence of reducible iron (III) metal sites on the adsorption of CO, CO2, propane, propene and propyne in the mesoporous metal–organic framework MIL-100. Phys Chem Chem Phys 13:11748–11756
Li A, Tang D, Song S, Song W, Ma L, Xu H, Kuang X, Wu L, Liu X (2016) A SERS-active sensor based on heterogeneous gold nanostar core–silver nanoparticle satellite assemblies for ultrasensitive detection of aflatoxin B1. Nanoscale 8:1873–1878
Li Y, Liu Z, Lu W, Zhao M, Xiao H, Hu T, Ma J, Zheng Z, Jia J, Wu H (2021) A label-free electrochemical aptasensor based on the core–shell Cu-MOF@ TpBD hybrid nanoarchitecture for the sensitive detection of PDGF-BB. Analyst 146:979–988
Li Y, Liu D, Zhu C, Shen X, Liu Y, You T (2020) Sensitivity programmable ratiometric electrochemical aptasensor based on signal engineering for the detection of aflatoxin B1 in peanut. J Hazard Mater 387:122001
Mairal T, Ozalp VC, Sánchez PL, Mir M, Katakis I, O’Sullivan CK (2008) Aptamers: molecular tools for analytical applications. Anal Bioanal Chem 390:989–1007
Miller JN, Miller JC (2000) Statistics and chemometrics for analytical chemistry. 4th edn. Pearson Education, Harlow, UK
Mo R, He L, Yan X, Su T, Zhou C, Wang Z, Hong P, Sun S, Li C (2018) A novel aflatoxin B1 biosensor based on a porous anodized alumina membrane modified with graphene oxide and an aflatoxin B1 aptamer. Electrochem Commun 95:9–13
Nejad ASM, Ghannad MS, Kamkar A (2014) Determination of aflatoxin B1 levels in Iranian and Indian spices by ELISA method. Toxin Rev 33:151–154
Oueslati S, Berrada H, Juan-García A, Mañes J, Juan C (2020) Multiple mycotoxin determination on Tunisian cereals-based food and evaluation of the population exposure. Food Anal Methods 13:1271–1281
Pereira CS, Cunha SC, Fernandes JO (2020) Validation of an enzyme-linked immunosorbent assay (ELISA) test kit for determination of aflatoxin B1 in corn feed and comparison with liquid-chromatography tandem mass spectrometry (LC-MS/MS) method. Food Anal Methods 13:1806–1816
Putzbach W, Ronkainen NJ (2013) Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors. Sens Rev 13:4811–4840
Qiao X, Xia F, Tian D, Chen P, Liu J, Gu J, Zhou C (2019) Ultrasensitive “signal-on” electrochemical aptasensor for assay of acetamiprid residues based on copper-centered metal-organic frameworks. Anal Chim Acta 1050:51–59
Ranjbar S, Shahrokhian S, Nurmohammadi F (2018) Nanoporous gold as a suitable substrate for preparation of a new sensitive electrochemical aptasensor for detection of Salmonella typhimurium. Sensor Actuat B Chem 255:1536–1544
Reddy K, Salleh B, Saad B, Abbas H, Abel C, Shier WT (2010) An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev 29:3–26
Roch OG, Blunden G, Coker RD, Nawaz S (1995) The validation of a solid phase clean-up procedure for the analysis of aflatoxins in groundnut cake using HPLC. Food Chem 52:93–98
Rodriguez-Carrasco Y, Izzo L, Gaspari A, Graziani G, Mañes J, Ritieni A (2018) Simultaneous determination of AFB1 and AFM1 in milk samples by ultra high performance liquid chromatography coupled to quadrupole orbitrap mass spectrometry. Beverage 4:43
Shekari Z, Zare HR, Falahati A (2017) Developing an impedimetric aptasensor for selective label–free detection of CEA as a cancer biomarker based on gold nanoparticles loaded in functionalized mesoporous silica films. J Electrochem Soc 164:B739
Sun L, Zhao Q (2018) Competitive horseradish peroxidase-linked aptamer assay for sensitive detection of Aflatoxin B1. Talanta 179:344–349
Tombelli S, Minunni M, Mascini M (2005) Analytical applications of aptamers. Biosens Bioelectron 20:2424–2434
Vikesland PJ, Wigginton KR (2010) Nanomaterial enabled biosensors for pathogen monitoring—a review. Environ Sci Technol 44:3656–3669
Wang D, Hu W, Xiong Y, Xu Y, Li CM (2015a) Multifunctionalized reduced graphene oxide-doped polypyrrole/pyrrolepropylic acid nanocomposite impedimetric immunosensor to ultra-sensitively detect small molecular aflatoxin B1. Biosens Bioelectron 63:185–189
Wang X, Lu X, Wu L, Chen J (2015b) 3D metal-organic framework as highly efficient biosensing platform for ultrasensitive and rapid detection of bisphenol A. Biosens Bioelectron 65:295–301
Xiao MW, Bai XL, Liu YM, Yang L, Liao X (2018) Simultaneous determination of trace aflatoxin B1 and Ochratoxin A by aptamer-based microchip capillary electrophoresis in food samples. J Chromatogr A 1569:222–228
Xu J, Li W, Shen P, Li Y, Li Y, Deng Y, Zheng Q, Liu Y, Ding Z, Li J, Zheng T (2017) Microfluidic fabrication of photonic encoding magnetized silica microspheres for aptamer-based enrichment of Ochratoxin A. Microchim Acta 184:3755–3763
Yang M, Liu G, Mehedi HM, Ouyang Q, Chen Q (2017) A universal sers aptasensor based on DTNB labeled GNTs/Ag core-shell nanotriangle and CS-Fe3O4 magnetic-bead trace detection of aflatoxin B1. Anal Chim Acta 986:122–130
Zheng W, Teng J, Cheng L, Ye Y, Pan D, Wu J, Xue F, Liu G, Chen W (2016) Hetero-enzyme-based two-round signal amplification strategy for trace detection of aflatoxin B1 using an electrochemical aptasensor. Biosens Bioelectron 80:574–581
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Fahime Jahangiri–Dehaghani declares that she has no conflict of interest. Hamid R. Zare declares that he has no conflict of interest. Zahra Shekari declares that she has no conflict of interest.
Informed Consent
Informed consent not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jahangiri–Dehaghani, F., Zare, H.R. & Shekari, Z. A Non-label Electrochemical Aptasensor Based on Cu Metal–Organic Framework to Measure Aflatoxin B1 in Wheat Flour. Food Anal. Methods 15, 192–202 (2022). https://doi.org/10.1007/s12161-021-02109-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02109-x