Abstract
Background
Salivary duct carcinoma with rhabdoid features (SDC-RF) is a recently-described salivary gland tumor that bears striking histologic similarity to lobular carcinoma of the breast. While this tumor has an apocrine phenotype that supports classification as a variant of SDC, it infrequently arises in association with conventional SDC. Furthermore, discohesive architecture can be seen in non-apocrine salivary carcinomas, raising the possibility that discohesive growth should define a separate entity. In this study, we aimed to perform comprehensive molecular profiling of SDC-RF to better understand its pathogenesis and classification.
Methods
We documented the clinicopathologic features of 9 cases of SDC-RF and performed immunostains including AR, GCDFP, and e-cadherin on all cases. We also performed targeted next generation sequencing (NGS) panels on 7 cases that had sufficient tissue available.
Results
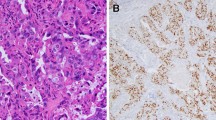
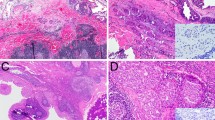
The SDC-RF represented 8 men and 1 woman with a median age of 67 years (range 63–83 years) and included 6 parotid, 2 buccal, and 1 submandibular primary. All tumors were uniformly composed of discohesive cells with abundant eosinophilic cytoplasm; signet-ring cell features were seen in 2 cases. All tumors were also positive for AR (100%) and GCDFP (100%), and 7 tumors (78%) displayed lost or abnormal e-cadherin. NGS highlighted concomitant PIK3CA and HRAS mutations in 4 tumors, with additional cases harboring TP53, PTEN, and AKT1 mutations. Furthermore, CDH1 alterations were seen in 6 cases, including a novel CDH1::CORO7 fusion. Among 5 patients with follow-up available, 3 (60%) developed local recurrence and widespread distant metastasis and died of disease at a median 20 months (range 10–48 months).
Conclusions
Overall, our findings confirm frequent CDH1 mutations and e-cadherin inactivation in SDC-RF, similar to discohesive tumors from other sites. We also highlight an apocrine molecular profile similar to conventional SDC. However, occasional AKT1 mutation and signet-ring features suggest SDC-RF may also be related to mucinous adenocarcinoma. As more salivary tumors with discohesive growth are identified, it may become clearer whether SDC-RF should remain in the SDC family or be recognized as a separate entity.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Kleinsasser O, Klein HJ, Hubner G. [Salivary duct carcinoma. A group of salivary gland tumors analogous to mammary duct carcinoma]. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1968;192(1):100–5.
Lewis JE, McKinney BC, Weiland LH, Ferreiro JA, Olsen KD. Salivary duct carcinoma. Clinicopathologic and immunohistochemical review of 26 cases. Cancer. 1996;77(2):223–30.
Brandwein-Gensler MS, Skalova A, Nagao T. Salivary duct carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D. International Agency for Research on Cancer., editor. Pathology and genetics of head and neck tumours. World Health Organization classification of tumours. Lyon: IARC; 2005. pp. 236–7.
Nagao T, Licitra L, Loening T, Vielh P, Williams MD. Salivary duct carcinoma. In: El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ, editors. WHO Classification of Head and Neck Tumours. Lyon: International Agency for Research on Cancer; 2017. pp. 173–4.
Chiosea SI, Williams L, Griffith CC, Thompson LD, Weinreb I, Bauman JE, et al. Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol. 2015;39(6):744–52.
Williams L, Thompson LD, Seethala RR, Weinreb I, Assaad AM, Tuluc M, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39(5):705–13.
Chiosea S, Agaimy A, Hellquist H, Nagao T, Simpson RHW, Van Herpen CM. Salivary duct carcinoma. In: WHO Classification of Tumours Editorial Board, editor. WHO Classification of Head and Neck Tumours. Lyon: International Agency for Research on Cancer; 2022.
Henley JD, Seo IS, Dayan D, Gnepp DR. Sarcomatoid salivary duct carcinoma of the parotid gland. Hum Pathol. 2000;31(2):208–13.
Michal M, Skalova A, Mukensnabl P. Micropapillary carcinoma of the parotid gland arising in mucinous cystadenoma. Virchows Arch. 2000;437(4):465–8.
Nagao T, Gaffey TA, Serizawa H, Iwaya K, Watanabe A, Yoshida T, et al. Sarcomatoid variant of salivary duct carcinoma: clinicopathologic and immunohistochemical study of eight cases with review of the literature. Am J Clin Pathol. 2004;122(2):222–31.
Nagao T, Gaffey TA, Visscher DW, Kay PA, Minato H, Serizawa H, et al. Invasive micropapillary salivary duct carcinoma: a distinct histologic variant with biologic significance. Am J Surg Pathol. 2004;28(3):319–26.
Simpson RH, Prasad AR, Lewis JE, Skalova A, David L. Mucin-rich variant of salivary duct carcinoma: a clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol. 2003;27(8):1070–9.
Simpson RH, Skalova A, Di Palma S, Leivo I. Recent advances in the diagnostic pathology of salivary carcinomas. Virchows Arch. 2014;465(4):371–84.
Rooper LM, Mansour M, Yonescu R, Oliai BR, Bishop JA, Westra WH. The Decline of Salivary Adenocarcinoma Not Otherwise Specified as a Tumor Entity: Reclassification Using Contemporary Immunohistochemical Profiling and Diagnostic Criteria. Am J Surg Pathol. 2020.
Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, et al. Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin Cancer Res. 2016;22(18):4623–33.
Dogan S, Ng CKY, Xu B, Kumar R, Wang L, Edelweiss M, et al. The repertoire of genetic alterations in salivary duct carcinoma including a novel HNRNPH3-ALK rearrangement. Hum Pathol. 2019;88:66–77.
Gargano SM, Senarathne W, Feldman R, Florento E, Stafford P, Swensen J, et al. Novel therapeutic targets in salivary duct carcinoma uncovered by comprehensive molecular profiling. Cancer Med. 2019;8(17):7322–9.
Karpinets TV, Mitani Y, Liu B, Zhang J, Pytynia KB, Sellen LD, et al. Whole-Genome Sequencing of Common Salivary Gland Carcinomas: Subtype-Restricted and Shared Genetic Alterations. Clin Cancer Res. 2021;27(14):3960–9.
Ku BM, Jung HA, Sun JM, Ko YH, Jeong HS, Son YI, et al. High-throughput profiling identifies clinically actionable mutations in salivary duct carcinoma. J Transl Med. 2014;12:299.
Luk PP, Weston JD, Yu B, Selinger CI, Ekmejian R, Eviston TJ, et al. Salivary duct carcinoma: Clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(Suppl 1):E1838-47.
Mueller SA, Gauthier MA, Blackburn J, Grady JP, Kraitsek S, Hajdu E, et al. Molecular patterns in salivary duct carcinoma identify prognostic subgroups. Mod Pathol. 2020;33(10):1896–909.
Saintigny P, Mitani Y, Pytynia KB, Ferrarotto R, Roberts DB, Weber RS, et al. Frequent PTEN loss and differential HER2/PI3K signaling pathway alterations in salivary duct carcinoma: Implications for targeted therapy. Cancer. 2018;124(18):3693–705.
Shimura T, Tada Y, Hirai H, Kawakita D, Kano S, Tsukahara K, et al. Prognostic and histogenetic roles of gene alteration and the expression of key potentially actionable targets in salivary duct carcinomas. Oncotarget. 2018;9(2):1852–67.
Jeong JS, Cho KJ, Kim D, Lee YS, Song JS. Genomic alteration in rare subtype of sarcomatoid salivary duct carcinoma. Pathol Res Pract. 2021;228:153678.
Kusafuka K, Onitsuka T, Muramatsu K, Miki T, Murai C, Suda T, et al. Salivary duct carcinoma with rhabdoid features: report of 2 cases with immunohistochemical and ultrastructural analyses. Head Neck. 2014;36(3):E28–35.
Kusafuka K, Kawasaki T, Maeda M, Yamanegi K, Baba S, Ito Y, et al. Salivary duct carcinoma with rhabdoid features: a salivary counterpart of pleomorphic lobular carcinoma of the breast. Histopathology. 2017;70(2):164–73.
Kusafuka K, Yamada H, Ishino K, Maeda M, Yamanegi K, Baba S, et al. Salivary Duct Carcinoma With Rhabdoid Features-No or Aberrant Expression of E-cadherin and Genetic Changes in CDH1: Immunohistochemical and Genetic Analyses of 17 Cases. Am J Surg Pathol. 2021;45(4):439–49.
Akaki M, Ishihara A, Nagai K, Naono H, Taguchi K, Yamamoto H, et al. Signet Ring Cell Differentiation in Salivary Duct Carcinoma with Rhabdoid Features: Report of Three Cases and Literature Review. Head Neck Pathol. 2021;15(1):341–51.
Otsuru M, Aoki T, Kondo Y, Ota Y, Sasaki M, Suzuki T, et al. Salivary Duct Carcinoma with Invasive Micropapillary and Rhabdoid Feature Arising in the Submandibular Gland. Tokai J Exp Clin Med. 2017;42(1):30–6.
Lei L, Van Staalduinen E, Troxell M, Ozawa MG, Zeineh M, Berry G. Mammary Lobular Carcinoma-Like Salivary Gland Carcinoma: Report of a Rare Case. Head Neck Pathol; 2021.
Rooper LM, Argyris PP, Thompson LDR, Gagan J, Westra WH, Jordan RC, et al. Salivary Mucinous Adenocarcinoma Is a Histologically Diverse Single Entity With Recurrent AKT1 E17K Mutations: Clinicopathologic and Molecular Characterization With Proposal for a Unified Classification. Am J Surg Pathol. 2021;45(10):1337–47.
Bishop JA, Gagan J, Baumhoer D, McLean-Holden AL, Oliai BR, Couce M, et al. Sclerosing Polycystic “Adenosis” of Salivary Glands: A Neoplasm Characterized by PI3K Pathway Alterations More Correctly Named Sclerosing Polycystic Adenoma. Head Neck Pathol. 2020;14(3):630–6.
Gondek LP, Zheng G, Ghiaur G, DeZern AE, Matsui W, Yegnasubramanian S, et al. Donor cell leukemia arising from clonal hematopoiesis after bone marrow transplantation. Leukemia. 2016;30(9):1916–20.
Palsgrove DN, Brosnan-Cashman JA, Giannini C, Raghunathan A, Jentoft M, Bettegowda C, et al. Subependymal giant cell astrocytoma-like astrocytoma: a neoplasm with a distinct phenotype and frequent neurofibromatosis type-1-association. Mod Pathol. 2018;31(12):1787–800.
Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61(4):514–23.
Bruner HC, Derksen PWB. Loss of E-Cadherin-Dependent Cell-Cell Adhesion and the Development and Progression of Cancer. Cold Spring Harb Perspect Biol. 2018;10(3).
Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108(29):11930–5.
Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, van Roy F, et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13(9):1919–25.
De Leeuw WJ, Berx G, Vos CB, Peterse JL, Van de Vijver MJ, Litvinov S, et al. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997;183(4):404–11.
Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol. 2015;1(1):23–32.
Massari G, Magnoni F, Favia G, Peradze N, Veronesi P, La Vecchia C, et al. Frequency of CDH1 Germline Mutations in Non-Gastric Cancers. Cancers (Basel). 2021;13(10).
Teo MY, Al-Ahmadie H, Seier K, Tully C, Regazzi AM, Pietzak E, et al. Natural history, response to systemic therapy, and genomic landscape of plasmacytoid urothelial carcinoma. Br J Cancer. 2021;124(7):1214–21.
Berger AC, Korkut A, Kanchi RS, Hegde AM, Lenoir W, Liu W, et al. A Comprehensive Pan-Cancer Molecular Study of Gynecologic and Breast Cancers. Cancer Cell. 2018;33(4):690–705 e9.
Liu Y, Sethi NS, Hinoue T, Schneider BG, Cherniack AD, Sanchez-Vega F, et al. Comparative Molecular Analysis of Gastrointestinal Adenocarcinomas. Cancer Cell. 2018;33(4):721–35. e8.
Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–13.
Droufakou S, Deshmane V, Roylance R, Hanby A, Tomlinson I, Hart IR. Multiple ways of silencing E-cadherin gene expression in lobular carcinoma of the breast. Int J Cancer. 2001;92(3):404–8.
Sarrio D, Moreno-Bueno G, Hardisson D, Sanchez-Estevez C, Guo M, Herman JG, et al. Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer. 2003;106(2):208–15.
Da Silva L, Parry S, Reid L, Keith P, Waddell N, Kossai M, et al. Aberrant expression of E-cadherin in lobular carcinomas of the breast. Am J Surg Pathol. 2008;32(5):773–83.
Dabbs DJ, Schnitt SJ, Geyer FC, Weigelt B, Baehner FL, Decker T, et al. Lobular neoplasia of the breast revisited with emphasis on the role of E-cadherin immunohistochemistry. Am J Surg Pathol. 2013;37(7):e1–11.
Bishop JA, Gagan J, Krane JF, Jo VY. Low-grade Apocrine Intraductal Carcinoma: Expanding the Morphologic and Molecular Spectrum of an Enigmatic Salivary Gland Tumor. Head Neck Pathol; 2020.
Hernandez-Prera JC, Saeed-Vafa D, Heidarian A, Gewandter K, Otto K, Wenig BM. Sclerosing Polycystic Adenoma: Conclusive Clinical and Molecular Evidence of Its Neoplastic Nature. Head Neck Pathol; 2021.
Hsieh MS, Lee YH, Jin YT, Kuo YJ. Clinicopathological study of intraductal carcinoma of the salivary gland, with emphasis on the apocrine type. Virchows Arch. 2020.
Skalova A, Baneckova M, Laco J, Di Palma S, Agaimy A, Ptakova N, et al. Sclerosing Polycystic Adenoma of Salivary Glands: A Novel Neoplasm Characterized by PI3K-AKT Pathway Alterations-New Insights Into a Challenging Entity. Am J Surg Pathol. 2022;46(2):268–80.
Lobo S, Benusiglio PR, Coulet F, Boussemart L, Golmard L, Spier I, et al. Cancer predisposition and germline CTNNA1 variants. Eur J Med Genet. 2021;64(10):104316.
Agaimy A, Baneckova M, Ihrler S, Mueller SK, Franchi A, Hartmann A, et al. ALK Rearrangements Characterize 2 Distinct Types of Salivary Gland Carcinomas: Clinicopathologic and Molecular Analysis of 4 Cases and Literature Review. Am J Surg Pathol. 2021;45(9):1166–78.
McLean-Holden AC, Rooper LM, Lubin DJ, Magliocca KR, Manucha V, Sadow PM, et al. Frankly Invasive Carcinoma Ex-intraductal Carcinoma: Expanding on an Emerging and Perplexing Concept in Salivary Gland Tumor Pathology. Head Neck Pathol; 2022.
Todorovic E, Dickson BC, Weinreb I. Salivary Gland Cancer in the Era of Routine Next-Generation Sequencing. Head Neck Pathol; 2020.
Di Palma S, Simpson RH, Marchio C, Skalova A, Ungari M, Sandison A, et al. Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology. 2012;61(4):629–43.
Takase S, Kano S, Tada Y, Kawakita D, Shimura T, Hirai H, et al. Biomarker immunoprofile in salivary duct carcinomas: clinicopathological and prognostic implications with evaluation of the revised classification. Oncotarget. 2017;8(35):59023–35.
Funding
This study was funded in part by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center. No external funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
LMR and JAB designed the study, contributed tumor samples, performed data collection and interpretation, and prepared the manuscript. JG performed data collection and interpretation. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethics approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (Johns Hopkins Medicine IRB 00176183 and UT Southwestern IRB 112017-073).
Consent to participate/for publication
The IRB-approved study did not require informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rooper, L.M., Gagan, J. & Bishop, J.A. Targeted molecular profiling of salivary duct carcinoma with rhabdoid features highlights parallels to other apocrine and discohesive neoplasms: which phenotype should drive classification?. Head and Neck Pathol 16, 1063–1072 (2022). https://doi.org/10.1007/s12105-022-01464-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-022-01464-3