Abstract
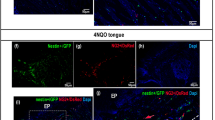
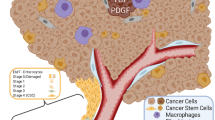
The microenvironment of oral cancer is highly dynamic and has been proved to affect tumor progression. Pericytes are blood vessels surrounding cells that have recently gained attention for their roles in vascular and cancer biology. The objective of the present study was to survey the scientific literature for conclusive evidence about whether pericytes are part of blood vessels in oral squamous cell carcinoma (OSCC) and their roles in the tumor microenvironment and clinical outcomes. A systematic electronic search was undertaken in Medline Ovid, PubMed, Web of Science, and Scopus. Eligibility criteria were: publications adopting in vivo models of OSCC that included pericyte detection and assessment by pericyte markers (e.g., α-smooth muscle actin, neuron-glial antigen 2 and platelet-derived growth factor receptor-β). The search yielded seven eligible studies (from 2008 to 2018). The markers most commonly used for pericyte detection were α-smooth muscle actin and neuron-glial antigen 2. The studies reviewed showed the presence of immature vessels exhibiting a reduction of pericyte coverage in OSCC and indicated that anti-cancer therapies could contribute to vessel normalization and pericyte regain. The pericyte population is significantly affected during OSCC development and cancer therapy. While these findings might suggest a role for pericytes in OSCC progression, the limited data available do not allow us to conclude whether they modify the tumor microenvironment and clinical outcome.


Similar content being viewed by others
References
Rouget C. Memoire sur le developpement, la structures et les proprietes des capillaires sanguins et lymphatiques. Archs Physiol Norm Pathol. 1873;5:603–33.
Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–21515.
Prazeres PH, Sena IFG, Borges IDT, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol. 2017;427:6–11.
Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: A review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269–86.
Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13.
Ayres-Sander CE, Lauridsen H, Maier CL, Sava P, Pober JS, Gonzalez AL. Transendothelial migration enables subsequent transmigration of neutrophils through underlying pericytes. PLoS ONE. 2013;8:1–12.
Kitahara H, Kajikawa S, Ishii Y, et al. The novel pathogenesis of retinopathy mediated by multiple RTK signals is uncovered in newly developed mouse model. EBioMedicine. 2018;31:190–201.
Shaw I, Rider S, Mullins J, Hughes J, Péault B. Pericytes in the renal vasculature: roles in health and disease. Nat Rev Nephrol. 2018;14:521–34.
Von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–9.
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–103.
Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–8.
Díaz-Flores L, Gutiérrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–69.
Park DY, Lee J, Kim J, et al. Plastic roles of pericytes in the blood–retinal barrier. Nat Commun. 2017;8:1–16.
Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:1–14.
Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–93.
Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WMF. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. 2003;162:183–93.
Sinha D, Chong L, George J, et al. Pericytes promote malignant ovarian cancer progression in mice and predict poor prognosis in serous ovarian cancer patients. Clin Cancer Res. 2006;22:1813–24.
Yonenaga Y, Mori A, Onodera H, et al. Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology. 2005;69:159–66.
Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000.
Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85:593–8.
Hosaka K, Yang Y, Seki T, et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci USA. 2016;113:5618–27.
Supakul S, Yao K, Ochi H, et al. Pericytes as a source of osteogenic cells in bone fracture healing. Int J Mol Sci. 2019;20:1–14.
Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–67.
Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–32.
Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology. 2005;7:452–64.
Lu C, Shahzad MM, Moreno-Smith M, et al. Targeting pericytes with a PDGF-B aptamer in human ovarian carcinoma models. Cancer Biol Ther. 2010;3:176–82.
Zhou W, Chen C, Shi Y, et al. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell. 2017;5:591–603.
Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Silva WN, Mintz A, Birbrair A. Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis. 2018;4:667–75.
Thijssen VL, Paulis YW, Nowak-Sliwinska P, et al. Targeting PDGF-mediated recruitment of pericytes blocks vascular mimicry and tumor growth. J Pathol. 2018;4:447–58.
Liu SY, Chang LC, Pan LF, Hung YJ, Lee CH, Shieh YS. Clinicopathologic significance of tumor cell-lined vessel and microenvironment in oral squamous cell carcinoma. Oral Oncol. 2008;44:277–85.
Margaritescu C, Simionescu C, Pirici D, Mogoanta L, Ciurea R, Stepan A. Immunohistochemical characterization of tumoral vessels in oral squamous cell carcinoma. Rom J Morphol Embryol. 2008;49:447–58.
Li C, Sun CJ, Fan JC, et al. Angiopoietin-2 expression is correlated with angiogenesis and overall survival in oral squamous cell carcinoma. Med Oncol. 2013;30:1–10.
Zhou H, Yang Y-H, Basile J. The Semaphorin 4D- Plexin-B1- RhoA signaling axis recruits pericytes and regulates vascular permeability through endothelial production of PDGF-B and ANGPTL4. Angiogenesis. 2014;17:261–74.
Chung TK, Warram J, Day KE, Hartman Y, Rosenthal EL. Time-dependent pretreatment with bevacuzimab increases tumor specific uptake of cetuximab in preclinical oral cavity cancer studies. Cancer Biol Ther. 2015;16:790–8.
Ludwig N, Yerneni SS, Razzo BM, Whiteside TL. Exosomes from HNSCC promote angiogenesis through reprogramming of endothelial cells. Mol Cancer Res. 2018;16:1798–808.
Prince AC, Patel NG, Moore LS, et al. Adjuvant anti-angiogenic therapy enhances chemotherapeutic uptake in a murine model of head and neck cancer. J Drug Target. 2018;27:193–200.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9.
Moola S, Munn Z, Tufanaru C, et al (2017) Checklist for analytical cross sectional studies. JBI Reviewer's Manual
Vanlandewijck M, He L, Mäe MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–80.
Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5.
Stratman AN, Malotte KM, Mahan RD, et al. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–101.
Velez DO, Tsui B, Goshia T, et al. 3D collagen architecture induces a conserved migratory and transcriptional response linked to vasculogenic mimicry. Nat Commun. 2017;8:1651.
Rudziak P, Ellis CG, Kowalewska PM. Role and molecular mechanisms of pericytes in regulation of leukocyte diapedesis in inflamed tissues. Mediators Inflamm. 2019. https://doi.org/10.1155/2019/4123605.
Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;7494:55–60.
Birbrair A, Borges IDT, Gilson Sena IF, et al. How plastic are pericytes? Stem Cells Dev. 2017;26:1013–9.
Asada N, Kunisaki Y, Pierce H, et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017;19:214–23.
Azevedo PO, Sena IFG, Andreotti JP, et al. Pericytes modulate myelination in the central nervous system. J Cell Physiol. 2018;8:5523–9.
Borges I, Sena I, Azevedo P, et al. Lung as a niche for hematopoietic progenitors. Stem Cell Rev. 2017;13:567–74.
Murgai M, Ju W, Eason M, et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med. 2018;23:1176–90.
Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110.
Guimaraes-Camboa N, Cattaneo P, Sun Y, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345–59.
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;16:2298–314.
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci. 2015;128:81–93.
Shivamallappa SM, Venkatraman NT, Shreedhar B, Mohanty L, Shenoy S. Role of angiogenesis in oral squamous cell carcinoma development and metastasis: an immunohistochemical study. Int J Oral Sci. 2011;3:216–24.
Brawer MK. Prostatic intraepithelial neoplasia: an overview. Rev Urol. 2005;7(Suppl 3):S11–S18.
Ozawa MG, Yao VJ, Chanthery YH, et al. Angiogenesis with pericyte abnormalities in a transgenic model of prostate carcinoma. Cancer. 2005;10:2104–15.
Basile JR, Castilho RM, Williams VP, Gutkind JS. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA. 2006;103:9017–22.
Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol Life Sci. 2015;72:1–10.
Xian X, Håkansson J, Ståhlberg A, et al. Pericytes limit tumor cell metastasis. J Clin Invest. 2006;116:642–51.
Ning X, Zhang H, Wang C, Song X. Exosomes released by gastric cancer cells induce transition of pericytes into cancer-associated fibroblasts. Med Sci Monit. 2018;24:2350–9.
Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;1:186–96.
Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401.
Voisin MB, Pröbstl D, Nourshargh S. Venular basement membranes ubiquitously express matrix protein low-expression regions. Am J Pathol. 2010;176:482–95.
Zhang L, Wang Y, Rashid MH. Malignant pericytes expressing GT198 give rise to tumor cells through angiogenesis. Oncotarget. 2017;8:51591–607.
Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2019;77:1745–70.
Maj E, Papiernik D, Wietrzyk J. Antiangiogenic cancer treatment: The great discovery and greater complexity. Int J Oncol. 2016;5:1773–844.
Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer. 2010;1:12–25.
Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Investig. 2003;111:1287–95.
Meng MB, Zaorsky NG, Deng L, et al. Pericytes: a double-edged sword in cancer therapy. Future Oncol. 2015;11:169–79.
Koonce NA, Griffin RJ, Dings RPM. Galectin-1 inhibitor otx008 induces tumor vessel normalization and tumor growth inhibition in human head and neck squamous cell carcinoma models. Int J Mol Sci. 2017;18:1–9.
Mondini M, Nizard M, Tran T, et al. Synergy of radiotherapy and a cancer vaccine for the treatment of HPV-associated head and neck cancer. Mol Cancer Ther. 2015;6:1336–455.
Nisancioglu MH, Betsholtz C, Genové G. The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-A blockade. Cancer Res. 2010;70:5109–15.
Acknowledgements
Research supported by the Brazilian National Council for Scientific and Technological Development (CNPq). T.A.S. and L.G.A. are research fellows of CNPq. I.B.V. and L.F.S. are the recipients of a fellowship granted by the Coordination of Improvement of Higher Education Personnel—Brazil (CAPES, Finance code 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valle, I.B., Schuch, L.F., da Silva, J.M. et al. Pericyte in Oral Squamous Cell Carcinoma: A Systematic Review. Head and Neck Pathol 14, 1080–1091 (2020). https://doi.org/10.1007/s12105-020-01188-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-020-01188-2