Abstract
Purpose
To investigate the role of PRDX2 in esophageal carcinoma (ESCA).
Methods
The expression of PRDX2 was detected in ESCA tissues. And PRDX2 expression in two ESCA cell lines was knocked down. Cell proliferation, metastasis and invasion were detected in these cells.
Results
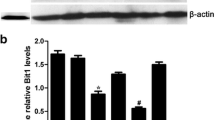
Here, we found that PRDX2 expression was significantly increased in ESCA tissues and was associated with a poor prognosis in ESCA patients. In addition, PRDX2 expression was significantly associated with pathological grading, infiltration degree and 5-year survival time in ESCA patients. Next, we knocked down PRDX2 expression by PRDX2-shRNA transfection in two ESCA cell lines, Eca-109 and TE-1. Proliferation analysis indicated that in vitro PRDX2 knockdown decreased growth and clone formation of ESCA cells. Scratch and transwell assays indicated that cell migration and invasion were significantly inhibited by PRDX2 knockdown. In addition, PRDX2 knockdown inhibited cell cycle of ESCA cells and down-regulated Cyclin D1-CDK4/6. Moreover, PRDX2 knockdown regulated proteins involved in mitochondrial-dependent apoptosis, including increased Bax and Caspase9/3 and decreased Bcl2. Mechanism investigation indicated that PRDX2 knockdown led to inactivation of Wnt/β-catenin and AKT pathways.
Conclusions
Our data suggest that PRDX2 may function as an oncogene in the development of ESCA via regulating Wnt/β-catenin and AKT pathways. Our study fills a gap in the understanding of the role of PRDX2 in ESCA and provides a potential target for ESCA treatment.








Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Kim T, Grobmyer SR, Smith R, Ben-David K, Ang D, Vogel SB, et al. Esophageal cancer–the five year survivors. J Surg Oncol. 2011;103(2):179–83.
Cavallin F, Scarpa M, Cagol M, Alfieri R, Ruol A, ChiarionSileni V, et al. Time to diagnosis in esophageal cancer: a cohort study. Acta Oncol. 2018;57(9):1179–84.
Bouzigues CI, Nguyen TL, Ramodiharilafy R, Claeson A, Tharaux PL, Alexandrou A. Regulation of the ROS response dynamics and organization to PDGF motile stimuli revealed by single nanoparticle imaging. Chem Biol. 2014;21(5):647–56.
Feng J, Fu Z, Guo J, Lu W, Wen K, Chen W, et al. Overexpression of peroxiredoxin 2 inhibits TGF-beta 1-induced epithelial-mesenchymal transition and cell migration in colorectal cancer. Molecular medicine reports. 2014;10(2):867–73.
Brown JD, Day AM, Taylor SR, Tomalin LE, Morgan BA, Veal EA. A peroxiredoxin promotes H2O2 signaling and oxidative stress resistance by oxidizing a thioredoxin family protein. Cell Rep. 2013;5(5):1425–35.
Yim MB, Chae HZ, Rhee SG, Chock PB, Stadtman ER. On the protective mechanism of the thiol-specific antioxidant enzyme against the oxidative damage of biomacromolecules. J Biol Chem. 1994;269(3):1621–6.
Fang J, Nakamura T, Cho D-H, Gu Z, Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson's disease. Proc Natl Acad Sci USA. 2007;104(47):18742–7.
Zhou S, Han Q, Wang R, Li X, Wang Q, Wang H, et al. PRDX2 protects hepatocellular carcinoma SMMC-7721 cells from oxidative stress. Oncol Lett. 2016;12(3):2217–21.
Kinnula VL, Lehtonen S, Sormunen R, Kaarteenaho-Wiik R, Kang SW, Rhee SG, et al. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol. 2002;196(3):316–23.
Lu W, Fu Z, Wang H, Feng J, Wei J, Guo J. Peroxiredoxin 2 is upregulated in colorectal cancer and contributes to colorectal cancer cells' survival by protecting cells from oxidative stress. Mol Cellular biochemistry. 2014;387(1–2):261–70.
Peng L, Wang R, Shang J, Xiong Y, Fu Z. Peroxiredoxin 2 is associated with colorectal cancer progression and poor survival of patients. Oncotarget. 2017;8(9):15057–700.
Kontostathi G, Zoidakis J, Makridakis M, Lygirou V, Mermelekas G, Papadopoulos T, et al. Cervical cancer cell line secretome highlights the roles of transforming growth factor-beta-induced protein ig-h3, peroxiredoxin-2, and NRF2 on cervical carcinogenesis. Biomed Res Int. 2017;2017:4180703.
Lu W, Fu Z, Wang H, Feng J, Wei J, Guo J. Peroxiredoxin 2 knockdown by RNA interference inhibits the growth of colorectal cancer cells by downregulating Wnt/beta-catenin signaling. Cancer Lett. 2014;343(2):190–9.
Cui L, Li F, Zhao Q, Li Z. Screening and verification of differentially expressed proteins from pancreatic cancer tissue. Chin J Chem. 2010;28:884–90.
Furuta J, Nobeyama Y, Umebayashi Y, Otsuka F, Kikuchi K, Ushijima T. Silencing of peroxiredoxin 2 and aberrant methylation of 33 CpG islands in putative promoter regions in human malignant melanomas. Cancer Res. 2006;66(12):6080–6.
Hong SH, Min C, Jun Y, Lee DJ, Kim SH, Park JH, et al. Silencing of peroxiredoxin II by promoter methylation is necessary for the survival and migration of gastric cancer cells. Exp Mol Med. 2018;50(2):e443.
Agrawal-Singh S, Isken F, Agelopoulos K, Klein H-U, Thoennissen NH, Koehler G, et al. Genome-wide analysis of histone H3 acetylation patterns in AML identifies PRDX2 as an epigenetically silenced tumor suppressor gene. Blood. 2012;119(10):2346–57.
Carnero A, Blancoaparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8(3):187.
Lin L-H, Xu Y-W, Huang L-S, Hong C-Q, Zhai T-T, Liao L-D, et al. Serum proteomic-based analysis identifying autoantibodies against PRDX2 and PRDX3 as potential diagnostic biomarkers in nasopharyngeal carcinoma. Clin Proteomics. 2017;14:6.
Nikoshkov A, Broliden K, Attarha S, Sviatoha V, Hellstrom A-C, Mints M, et al. Expression pattern of the PRDX25 RAB1A, RAB1B, RAB5A and RAB25 genes in normal and cancer cervical tissues. Int J Oncol. 2015;46(1):107–12.
Stresing V, Baltziskueta E, Rubio N, Blanco J, Arriba MC, Valls J, et al. Peroxiredoxin 2 specifically regulates the oxidative and metabolic stress response of human metastatic breast cancer cells in lungs. Oncogene. 2013;32(6):724–35.
Shiota M, Izumi H, Miyamoto N, Onitsuka T, Kashiwagi E, Kidani A, et al. Ets regulates peroxiredoxin1 and 5 expressions through their interaction with the high-mobility group protein B1. Cancer Sci. 2008;99(10):1950–9.
Zhang S, Fu Z, Wei J, Guo J, Liu M, Du K. Peroxiredoxin 2 is involved in vasculogenic mimicry formation by targeting VEGFR2 activation in colorectal cancer. Med Oncol. 2015;32(1):414.
Diao S, Zhang J-F, Wang H, He M-I, Lin MC, Chen Y, et al. Proteomic identification of microRNA-122a target proteins in hepatocellular carcinoma. Proteomics. 2010;10(20):3723–31.
Jiang Q-Q, Liu B, Yuan T. MicroRNA-16 inhibits bladder cancer proliferation by targeting cyclin D1. Asian Pac J Cancer Prev. 2013;14(7):4127–30.
Guidolin D, Agnati LF, Tortorella C, Marcoli M, Maura G, Albertin G, et al. Neuroglobin as a regulator of mitochondrial-dependent apoptosis: a bioinformatics analysis. Int J Mol Med. 2014;33(1):111–6.
Wang R, Wei J, Zhang S, Wu X, Guo J, Liu M, et al. Peroxiredoxin 2 is essential for maintaining cancer stem cell-like phenotype through activation of Hedgehog signaling pathway in colon cancer. Oncotarget. 2016;7(52):86816–28.
Fatima I, El-Ayachi I, Taotao L, Lillo MA, Krutilina RI, Seagroves TN, et al. The natural compound Jatrophone interferes with Wnt/β-catenin signaling and inhibits proliferation and EMT in human triple-negative breast cancer. PLoS ONE. 2017;12(12):e0189864.
Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–83.
He R, Zhang FH, Shen N. LncRNA FEZF1-AS1 enhances epithelial–mesenchymal transition (EMT) through suppressing E-cadherin and regulating WNT pathway in non-small cell lung cancer (NSCLC). Biomed Pharmacother. 2017;95:331–8.
Acknowledgements
This study was funded by National Natural Science Foundation of China (Foundation Number: 81502508 and 81500496).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ALF, XH, XM, ZC, QL, WS, HD, JZ and ZY. The first draft of the manuscript was written by ALF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This work has been approved by the ethical committees at Shandong Provincial Hospital affiliated to Shandong University.
Informed consent
All patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, A.L., Han, X., Meng, X. et al. PRDX2 plays an oncogenic role in esophageal squamous cell carcinoma via Wnt/β-catenin and AKT pathways. Clin Transl Oncol 22, 1838–1848 (2020). https://doi.org/10.1007/s12094-020-02323-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02323-9