Abstract
A brief review of tumor immunotherapies shows significant advancements in academic research and preclinical studies. Analysis of different immune cell pathways, including macrophage activation, natural killer cells, and dendritic cell presentation show promising clinical results when targeted with different nanoparticle polymer and gold materials. Following a brief discussion on immuno-oncology successes, detailed results are discussed in macrophage activation, dendritic cell presentation, and lysis of tumor cells with natural killer cells. Common targets include tumor-associated macrophages and induction of the proinflammatory phenotype, dual targeting of cell and humoral immunity with dendritic cells, and creating chimeric antigen receptors on natural killer cells. An analysis of the results shows a variety of nanoparticle synthesis methods are required depending on drug type and tissue type affected by tumors. Future research is discussed in conjunction with a brief analysis of completed clinical trial data on cancer therapies using nanoparticles to date. Although paclitaxel-loaded albumin nanoparticles are most frequently studied, academic research shows there may be additional mechanisms of action to elicit anti-tumor activity.

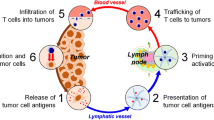
(Adapted from a detailed timeline from Oiseth et al.)


Similar content being viewed by others
References
Kapadia CH, Perry JL, Tian S, Luft JC, DeSimone JM. Nanoparticulate immunotherapy for cancer. J Control Release. 2015;219:167–80.
Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat. 2017;3(10):250.
Cousin S, Seneschal J, Italiano A. Toxicity profiles of immunotherapy. Pharmacol Ther. 2018;181:91–100.
Van Woensel M, Mathivet T, Wauthoz N, Rosière R, Garg AD, Agostinis P, Mathieu V, Kiss R, Lefranc F, Boon L, et al. Sensitization of glioblastoma tumor micro- environment to chemo- and immunotherapy by galectin-1 intranasal knock-down strategy. Sci Rep. 2017;7(1):1–14.
Lin JC, Liu CL, Lee JJ, Liu TP, Ko WC, Huang YC, Wu CH, Chen YJ. Sorafenib induces autophagy and suppresses activation of human macrophage. Int Immunopharmacol. 2013;15(2):333–9.
Chen P, Cescon M, Bonaldo P. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy. 2014;10(2):192–200.
Zhan X, Jia L, Niu Y, Qi H, Chen X, Zhang Q, Zhang J, Wang Y, Dong L, Wang C. Targeted depletion of tumour-associated macrophages by an alendronate-glucomannan conjugate for cancer immunotherapy. Biomaterials. 2014;35(38):10046–57.
Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy R. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10(31):4488.
Li C, Zhang X, Chen Q, Zhang J, Li W, Hu H, Zhao X, Qiao M, Chen D. Synthetic polymeric mixed micelles targeting lymph nodes trigger enhanced cellular and humoral immune responses. ACS Appl Mater Interfaces. 2018;10(3):2874–89.
Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381(1):244–51.
Pitchaimani A, Duong T, Nguyen T, Aryal S. Biomaterials natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials. 2018;160:124–37.
Zhang Y, Li N, Suh H, Irvine DJ. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9(1):6.
Kwong B, Liu H, Irvine DJ. Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials. 2011;32(22):5134–47.
Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273–86.
Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomed Nanotechnol Biol Med. 2018;14(2):237–46.
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12.
Pillai G. Nanomedicines for cancer therapy: an update of FDA approved and those under various stages of development. SOJ Pharm Pharm Sci. 2014;1:13.
Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61(5):1591–602.
Mocan T, Matea C, Tabaran F, Iancu C, Orasan R, Mocan L. In vitro administration of gold nanoparticles functionalized with MUC-1 protein fragment generates anticancer vaccine response via macrophage activation and polarization mechanism. J Cancer. 2015;6(6):583–92.
Jin H, Wan C, Zou Z, Zhao G, Zhang L, Geng Y, Chen T, Huang A, Jiang F, Feng J-P, et al. Tumor ablation and therapeutic immunity induction by an injectable peptide hydrogel. ACS Nano. 2018;12:3295–310 (acsnano.7b08148).
Sehgal K, Ragheb R, Fahmy TM, Dhodapkar MV, Dhodapkar KM. Nanoparticle-mediated combinatorial targeting of multiple human dendritic cell (DC) subsets leads to enhanced T cell activation via IL-15–dependent DC crosstalk. J Immunol. 2014;193(5):2297–305.
Jiao P, Otto M, Geng Q, Li C, Li F, Butch ER, Snyder SE, Zhou H, Yan B. Enhancing both CT imaging and natural killer cell-mediated cancer cell killing by a GD2-targeting nanoconstruct. J Mater Chem B. 2016;4(3):513–20.
Siegler EL, Kim YJ, Chen X, Siriwon N, Mac J, Rohrs JA, Bryson PD, Wang P. Combination cancer therapy using chimeric antigen receptor-engineered natural killer cells as drug carriers. Mol Ther. 2017;25(12):2607–19.
Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy—advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:1–7.
Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, MacArulla T, Lee KH, Cunningham D, Blanc JF, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–57.
Hao RT, Chen J, Zhao LH, Liu C, Wang OC, Huang GL, Zhang XH, Zhao J. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur J Surg Oncol. 2012;38(8):718–24.
Northfelt DW, Dueck AC, Flynn TP, Sander PJ, Stella PJ, Melnik M, Pavey ES, EP. Phase II trial combining nab-paclitaxel (NP), gemcitabine (G), and bevacizumab (B) in patients (Pts) with metastatic breast cancer (MBC): NCCTG N0735 (Abstract). J Clin Oncol. 2011; 29(Suppl 15):A-1126.
Roy V, Laplant BR, Gross GG, Bane CL, Palmieri FM. Phase II trial of weekly nab (nanoparticle albumin-bound)-paclitaxel (nab-paclitaxel) (Abraxane®) in combination with gemcitabine in patients with metastatic breast cancer (N0531). Ann Oncol. 2009;20(3):449–53.
Yardley D, Burris H, Peacock N, Raefsky E, Melnik M, Inhorn R, Shipley D, Hainsworth J. A Pilot study of adjuvant nanoparticle albumin-bound (Nab) paclitaxel and cyclophosphamide, with trastuzumab in HER2-positive patients, in the treatment of early-stage breast cancer. Breast Cancer Res Treat. 2010;123(2):471–5.
Acknowledgements
The authors would like to thank Dr. Marissa Gray at Stevens Institute of Technology for supporting this review paper and guiding the focus of research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest are reported by the authors.
Ethical approval
No human studies were conducted in the development of this review paper.
Informed consent
Informed consent is not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Savitsky, K., Yu, X. Combined strategies for tumor immunotherapy with nanoparticles. Clin Transl Oncol 21, 1441–1449 (2019). https://doi.org/10.1007/s12094-019-02081-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02081-3