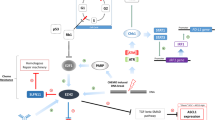
Abstract
Despite decades of research, prognosis for SCLC patients remains poor, and treatment options limited. SCLC is an immunogenic tumor with high somatic mutation rates due to tobacco exposure resulting in potential neo-antigens, the presence of suppressed immune responses, and occurrence of paraneoplastic disorders. The use of T cell immune-checkpoint inhibitors (anti-PD1: nivolumab, pembrolizumab; anti-PD-L1: atezolizumab, durvalumab; anti-CTLA-4: ipilimumab, tremelimumab) have shown promising antitumor activity with the potential to prolong survival in SCLC patients. In fact, atezolizumab when combined with chemotherapy has achieved the milestone of being the first drug to improve survival in patients with newly diagnosed extensive-stage SCLC. Other immunotherapeutic approaches evaluated in clinical trials for SCLC include the use of cytokines, cancer vaccines, antiganglioside therapies, TLR9 inhibition, anti-Notch signaling, and anti-CD47. This review discusses the rationale and clinical evidence of immunotherapy in SCLC, the conflictive clinical results of novel immunotherapeutic agents and combinatorial therapies under evaluation in SCLC patients.


Similar content being viewed by others
References
Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now?—a review. Transl Lung Cancer Res. 2016;5(1):26–38.
George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. https://doi.org/10.1038/nature14664.
Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, et al. Treatment of small-cell lung cancer: American society of clinical oncology endorsement of the American college of chest physicians guideline. J Clin Oncol. 2015;33(34):4106–11. https://doi.org/10.1200/jco.2015.63.7918.
Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl_6):99–105. https://doi.org/10.1093/annonc/mdt178.
Artal Cortés Á, Dómine Gómez M, Font Pous A, García Campelo R, Cobo Dolls M, Isla Casado D. SEOM clinical guidelines for the treatment of small-cell lung cancer. Clin Transl Oncol. 2010;12(1):27–31. https://doi.org/10.1007/s12094-010-0463-2.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. https://doi.org/10.1016/j.immuni.2013.07.012.
Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc Natl Acad Sci USA. 1996;93(10):4529–36.
Maddison P, Newsom-Davis J, Mills KR, Souhami RL. Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma. Lancet. 1999;353(9147):117–8. https://doi.org/10.1016/S0140-6736(05)76153-5.
Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: beyond traditional treatment approaches. J Thorac Oncol. 2013;8(5):587–98. https://doi.org/10.1097/JTO.0b013e318286cf88.
Wang W, Hodkinson P, McLaren F, Mackean MJ, Williams L, Howie SEM, et al. Histologic assessment of tumor-associated CD45(+) cell numbers is an independent predictor of prognosis in small cell lung cancer. Chest. 2013;143(1):146–51. https://doi.org/10.1378/chest.12-0681.
Koyama K, Kagamu H, Miura S, Hiura T, Miyabayashi T, Itoh R, et al. Reciprocal CD41 T-cell balance of effector CD62Llow CD41 and CD62LhighCD251 CD41 regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14(21):6770–9. https://doi.org/10.1158/1078-0432.Ccr-08-1156.
Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10(3):426–30. https://doi.org/10.1097/JTO.0000000000000414.
Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104. https://doi.org/10.1038/ng.2396. https://www.nature.com/articles/ng.2396#supplementary-information.
Weiss GJ, Byron SA, Aldrich J, Sangal A, Barilla H, Kiefer JA, et al. A prospective pilot study of genome-wide exome and transcriptome profiling in patients with small cell lung cancer progressing after first-line therapy. PLoS One. 2017;12(6):e0179170. https://doi.org/10.1371/journal.pone.0179170.
Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33(5):853. https://doi.org/10.1016/j.ccell.2018.04.001.
Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725. https://doi.org/10.1038/nrc.2017.87. https://www.nature.com/articles/nrc.2017.87#supplementary-information.
Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14:549. https://doi.org/10.1038/nrclinonc.2017.71.
Yarchoan M, Johnson III BA, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017;17:209. https://doi.org/10.1038/nrc.2016.154.
Efremova M, Finotello F, Rieder D, Trajanoski Z. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front Immunol. 2017. https://doi.org/10.3389/fimmu.2017.01679.
Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res. 2016;22(4):813–20. https://doi.org/10.1158/1078-0432.Ccr-15-1678.
Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–608. https://doi.org/10.1158/1535-7163.Mct-17-0386.
Doyle A, Martin WJ, Funa K, Gazdar A, Carney D, Martin SE, et al. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985;161(5):1135–51.
He Y, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, Yu H, et al. MHC class II expression in lung cancer. Lung Cancer. 2017;112:75–80. https://doi.org/10.1016/j.lungcan.2017.07.030.
Schalper KA, Carvajal-Hausdorf DE, McLaughlin JF, Altan M, Chiang AC, Velcheti V et al. Objective measurement and significance of PD-L1, B7-H3, B7-H4 and TILs in small cell lung cancer (SCLC). J Clin Oncol. 2016;34(15_suppl):8566. https://doi.org/10.1200/jco.2016.34.15_suppl.8566.
Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3 Pt 1):878–87. https://doi.org/10.1158/1078-0432.CCR-05-2013.
Rivalland G, Walkiewicz M, Wright GM, John T. Small cell lung cancer: the immune microenvironment and prognostic impact of checkpoint expression. J Clin Oncol. 2017;35(15_suppl):8569. https://doi.org/10.1200/jco.2017.35.15_suppl.8569.
Clamon G, Herndon J, Perry MC, Ozer H, Kreisman H, Maher T, et al. Interleukin-2 activity in patients with extensive small-cell lung cancer: a phase II trial of Cancer and Leukemia Group B. J Natl Cancer Inst. 1993;85(4):316–20.
Clamon G, Herndon J, Akerley W, Green M. Subcutaneous interleukin-2 as initial therapy for patients with extensive small cell lung cancer. Lung Cancer. 1998;19(1):25–9. https://doi.org/10.1016/S0169-5002(97)00070-6.
Zarogoulidis K, Ziogas E, Papagiannis A, Charitopoulos K, Dimitriadis K, Economides D, et al. Interferon alpha-2a and combined chemotherapy as first line treatment in SCLC patients: a randomized trial. Lung Cancer. 1996;15(2):197–205.
Zarogoulidis K, Ziogas E, Boutsikou E, Zarogoulidis P, Darwiche K, Kontakiotis T, et al. Immunomodifiers in combination with conventional chemotherapy in small cell lung cancer: a phase II, randomized study. Drug Des, Dev Ther. 2013;7:611–7. https://doi.org/10.2147/dddt.S43184.
Mattson K, Niiranen A, Pyrhonen S, Holsti LR, Holsti P, Kumpulainen E, et al. Natural interferon alfa as maintenance therapy for small cell lung cancer. Eur J Cancer. 1992;28a(8–9):1387–91.
Jett JR, Maksymiuk AW, Su JQ, Mailliard JA, Krook JE, Tschetter LK, et al. Phase III trial of recombinant interferon gamma in complete responders with small-cell lung cancer. J Clin Oncol. 1994;12(11):2321–6. https://doi.org/10.1200/jco.1994.12.11.2321.
Kelly K, Crowley JJ, Bunn PA Jr, Hazuka MB, Beasley K, Upchurch C, et al. Role of recombinant interferon alfa-2a maintenance in patients with limited-stage small-cell lung cancer responding to concurrent chemoradiation: a Southwest Oncology Group study. J Clin Oncol. 1995;13(12):2924–30. https://doi.org/10.1200/jco.1995.13.12.2924.
van Zandwijk N, Groen HJ, Postmus PE, Burghouts JT, ten Velde GP, Ardizzoni A, et al. Role of recombinant interferon-gamma maintenance in responding patients with small cell lung cancer. A randomised phase III study of the EORTC Lung Cancer Cooperative Group. Eur J Cancer. 1997;33(11):1759–66.
Pillai RN, Aisner J, Dahlberg SE, Rogers JS, DiPaola RS, Aisner S, et al. Interferon alpha plus 13-cis-retinoic acid modulation of BCL-2 plus paclitaxel for recurrent small-cell lung cancer (SCLC): an Eastern Cooperative Oncology Group study (E6501). Cancer Chemother Pharmacol. 2014;74(1):177–83. https://doi.org/10.1007/s00280-014-2427-7.
Shengle Z, Carlos C-C, Zhang S, Reuter VE, Sucharita A, Bradley HW, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73(1):42–9. https://doi.org/10.1002/(sici)1097-0215(19970926)73:1%3c42:aid-ijc8%3e3.0.co;2-1.
Krug LM, Ragupathi G, Hood C, Kris MG, Miller VA, Allen JR, et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10(18 Pt 1):6094–100. https://doi.org/10.1158/1078-0432.CCR-04-0482.
Krug LM, Ragupathi G, Ng KK, Hood C, Jennings HJ, Guo Z, et al. Vaccination of small cell lung cancer patients with polysialic acid or N-propionylated polysialic acid conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10(3):916–23.
Krug LM, Ragupathi G, Hood C, George C, Hong F, Shen R, et al. Immunization with N-propionyl polysialic acid-KLH conjugate in patients with small cell lung cancer is safe and induces IgM antibodies reactive with SCLC cells and bactericidal against group B meningococci. Cancer Immunol Immunother. 2012;61(1):9–18. https://doi.org/10.1007/s00262-011-1083-6.
Chada S, Mhashilkar A, Roth JA, Gabrilovich D. Development of vaccines against self-antigens: the p53 paradigm. Curr Opin Drug Discov Devel. 2003;6(2):169–73.
Vierboom MP, Nijman HW, Offringa R, van der Voort EI, van Hall T, van den Broek L, et al. Tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. J Exp Med. 1997;186(5):695–704.
Zwaveling S, Vierboom MP, Ferreira Mota SC, Hendriks JA, Ooms ME, Sutmuller RP, et al. Antitumor efficacy of wild-type p53-specific CD4(+) T-helper cells. Cancer Res. 2002;62(21):6187–93.
Chiappori AA, Soliman H, Janssen WE, Antonia SJ, Gabrilovich DI. INGN-225: a dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: observed association between immune response and enhanced chemotherapy effect. Expert Opin Biol Ther. 2010;10(6):983–91. https://doi.org/10.1517/14712598.2010.484801.
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9. https://doi.org/10.1038/nature10673.
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. https://doi.org/10.1038/nri3405.
Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015;26(9):1813–23. https://doi.org/10.1093/annonc/mdv209.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252. https://doi.org/10.1038/nrc3239.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331.
Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. https://doi.org/10.1093/annonc/mds213.
Arriola E, Wheater M, Galea I, Cross N, Maishman T, Hamid D, et al. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J Thorac Oncol. 2016;11(9):1511–21. https://doi.org/10.1016/j.jtho.2016.05.028.
Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–8. https://doi.org/10.1200/JCO.2016.67.6601.
Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–95. https://doi.org/10.1016/s1470-2045(16)30098-5.
Hellmann MD, Ott PA, Zugazagoitia J, Ready NE, Hann CL, De Braud FG, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): first report of a randomized expansion cohort from CheckMate 032. J Clin Oncol. 2017;35(15_suppl):8503. https://doi.org/10.1200/jco.2017.35.15_suppl.8503.
National Comprehensive Cancer Network. Small Cell Lung Cancer (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed 15 Jun 2018.
Horn L, Reck M, Gettinger SN, Spigel DR, Antonia SJ, Rupnow BA, et al. CheckMate 331: An open-label, randomized phase III trial of nivolumab versus chemotherapy in patients (pts) with relapsed small cell lung cancer (SCLC) after first-line platinum-based chemotherapy (PT-DC). J Clin Oncol. 2016;34(15_suppl):TPS8578. https://doi.org/10.1200/jco.2016.34.15_suppl.tps8578.
Ready N, Owonikoko TK, Postmus PE, Reck M, Peters S, Pieters A, et al. CheckMate 451: A randomized, double-blind, phase III trial of nivolumab (nivo), nivo plus ipilimumab (ipi), or placebo as maintenance therapy in patients (pts) with extensive-stage disease small cell lung cancer (ED-SCLC) after first-line platinum-based doublet chemotherapy (PT-DC). J Clin Oncol. 2016;34(15_suppl):TPS8579. https://doi.org/10.1200/jco.2016.34.15_suppl.tps8579.
De Ruysscher D, Pujol JL, Popat S, Reck M, Le Pechoux C, Liston A, et al. STIMULI: a randomised open-label phase II trial of consolidation with nivolumab and ipilimumab in limited-stage SCLC after standard of care chemo-radiotherapy conducted by ETOP and IFCT. Ann Oncol. 2016;27(suppl_6):1430TiP-TiP. https://doi.org/10.1093/annonc/mdw389.08.
Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 Study. J Clin oncol. 2017;35(34):3823–9. https://doi.org/10.1200/JCO.2017.72.5069.
Chung HC, Lopez-Martin JA, Kao SC-H, Miller WH, Ros W, Gao B et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol. 2018;36(15_suppl):8506-. https://doi.org/10.1200/jco.2018.36.15_suppl.8506.
Gadgeel SM, Pennell NA, Fidler MJ, Halmos B, Bonomi P, Stevenson J, et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol. 2018. https://doi.org/10.1016/j.jtho.2018.05.002.
Rudin CM, Shen L, Pietanza MC. KEYNOTE-604: Phase 3 trial of pembrolizumab plus etoposide/platinum (EP) for first-line treatment of extensive stage small-cell lung cancer (ES-SCLC). Ann Oncol. 2017;28(suppl_5):mdx386.008. https://doi.org/10.1093/annonc/mdx386.008.
Sequist LV, Chiang A, Gilbert J, Gordon M, Conkling PR, Thompson D, et al. Clinical activity, safety and predictive biomarkers results from a phase Ia atezolizumab (atezo) trial in extensive-stage small cell lung cancer (ES-SCLC). Ann Oncol. 2016;27(suppl_6):1425PD-PD. https://doi.org/10.1093/annonc/mdw389.03.
Pujol JL, Greillier L, Audigier Valette C, Moro-Sibilot D, Uwer L, Hureaux J, et al. A randomized non-comparative phase II study of anti–PD-L1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: results from the IFCT-1603 trial. Ann Oncol. 2018;29(Suppl 8):1664O. https://doi.org/10.1093/annonc/mdy298.
Horn L, Reck M, Mok TSK, Johnson M, Waterkamp D, Lam S, et al. A Phase III study of atezolizumab with carboplatin plus etoposide in patients with extensive-stage small cell lung cancer (IMpower133). Ann Oncol. 2016;27(suppl_6):1431TiP-TiP. https://doi.org/10.1093/annonc/mdw389.09.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9. https://doi.org/10.1056/nejmoa1809064.
Pawel JV, Vynnychenko I, Jiang H, Huang Y, Dennis PA. A phase II, open-label, multi-arm study of novel combinations of immunotherapies or DDR inhibitors in platinum-refractory, extensive disease small-cell lung cancer (ED-SCLC): BALTIC. J Clin Oncol. 2017;35(15_suppl):TPS8585-TPS. https://doi.org/10.1200/jco.2017.35.15_suppl.tps8585.
Paz-Ares L, Jiang H, Huang Y, Dennis P. CASPIAN: phase 3 study of first-line durvalumab ± tremelimumab + platinum-based chemotherapy vs chemotherapy alone in ED-SCLC. J Thorac Oncol. 2017;12(11):S2398. https://doi.org/10.1016/j.jtho.2017.11.015.
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–20. https://doi.org/10.1158/1078-0432.CCR-16-3215.
Krebs M, Ross K, Kim S, De Jonge M, Barlesi F, Postel-Vinay S, et al. An open-label, multitumor phase II basket study of olaparib and durvalumab (MEDIOLA): results in patients with relapsed SCLC. J Thorac Oncol. 2017;12(11):S2044–S5. https://doi.org/10.1016/j.jtho.2017.09.1040.
Schmidt M, Hagner N, Marco A, Konig-Merediz SA, Schroff M, Wittig B. Design and structural requirements of the potent and safe TLR-9 agonistic immunomodulator MGN1703. Nucleic Acid Ther. 2015;25(3):130–40. https://doi.org/10.1089/nat.2015.0533.
Thomas M, Ponce-Aix S, Navarro Mendivil A, Riera Knorrenschild J, Schmidt M, Krikow M, et al. Top-line data from the randomized phase 2 IMPULSE study in small-cell lung cancer (SCLC): Immunotherapeutic maintenance treatment with lefitolimod. Ann Oncol. 2017;28(suppl_5):mdx386. https://doi.org/10.1093/annonc/mdx386.
Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7(302):302ra136. https://doi.org/10.1126/scitranslmed.aac9459.
Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51. https://doi.org/10.1016/s1470-2045(16)30565-4.
Carbone DP, Morgensztern D, Moulec SL, Santana-Davila R, Ready N, Hann CL, et al. Efficacy and safety of rovalpituzumab tesirine in patients with DLL3-expressing, ≥ 3rd line small cell lung cancer: results from the phase 2 TRINITY study. J Clin Oncol. 2018;36(15_suppl):8507. https://doi.org/10.1200/jco.2018.36.15_suppl.8507.
Daniel DB, Rudin CM, Hart L, Spigel DR, Edelman MJ, Goldschmidt J, et al. Results of a randomized, placebo-controlled, phase 2 study of tarextumab (TRXT, anti-Notch2/3) in combination with etoposide and platinum (EP) in patients (pts) with untreated extensive-stage small-cell lung cancer (ED-SCLC). Ann Oncol. 2017;28(suppl_5):mdx386.004. https://doi.org/10.1093/annonc/mdx386.004.
Chu QSC, Markman B, Leighl N, Krug L, Rudin C, Lathers D, et al. A phase 1/2 trial of a monoclonal antibody targeting fucosyl GM1 in relapsed/refractory small cell lung cancer (SCLC): safety and preliminary efficacy. Ann Oncol. 2016;27(suppl_6):1427PD. https://doi.org/10.1093/annonc/mdw389.05.
Chu QSC, van Herpen C, Leighl NB, Markman B, Clarke S, Juergens RA, et al. Initial results of BMS-986012, a first-in-class fucosyl-GM1 mAb, in combination with nivolumab, in pts with relapsed/refractory (rel/ref) small-cell lung cancer (SCLC). Ann Oncol. 2017;28(suppl_5):mdx386.002. https://doi.org/10.1093/annonc/mdx386.002.
Mueller BM, Romerdahl CA, Gillies SD, Reisfeld RA. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol. 1990;144(4):1382–6.
Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr Cancer Drug Targets. 2010;10(2):200–9.
Dhillon S. Dinutuximab: first global approval. Drugs. 2015;75(8):923–7. https://doi.org/10.1007/s40265-015-0399-5.
Castel V, Segura V, Canete A. Treatment of high-risk neuroblastoma with anti-GD2 antibodies. Clin Transl Oncol. 2010;12(12):788–93. https://doi.org/10.1007/s12094-010-0600-y.
Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPalpha axis. Eur J Cancer. 2017;76:100–9. https://doi.org/10.1016/j.ejca.2017.02.013.
Xiang Y-R, Liu L. Eating cancer cells by blocking CD47 signaling: cancer therapy by targeting the innate immune checkpoint. Cancer Transl Med. 2017;3(6):200–8. https://doi.org/10.4103/ctm.ctm_26_17.
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109(17):6662–7. https://doi.org/10.1073/pnas.1121623109.
Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest. 2016;126(7):2610–20. https://doi.org/10.1172/JCI81603.
Navarro A, Felip E. Pembrolizumab in advanced pretreated small cell lung cancer patients with PD-L1 expression: data from the KEYNOTE-028 trial: a reason for hope? Transl Lung Cancer Res. 2017;6(Suppl 1):S78–83. https://doi.org/10.21037/tlcr.2017.10.04.
Giaccone G, Debruyne C, Felip E, Chapman PB, Grant SC, Millward M, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol. 2005;23(28):6854–64. https://doi.org/10.1200/jco.2005.17.186.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AC has received honorary/consulting fees from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche/Genentech, Eli Lilly and Company, Novartis, Merck Sharp & Dohme, and Bristol-Myers Squibb. The rest of the authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not applicable to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Calles, A., Aguado, G., Sandoval, C. et al. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol 21, 961–976 (2019). https://doi.org/10.1007/s12094-018-02011-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-02011-9