Abstract
Purpose
Accumulating evidence has elucidated that the interaction between cancer cells and M2 macrophages plays an important role in the tumorigenesis of hepatocellular carcinoma (HCC). However, the mechanism connecting tumor-derived exosomes, M2 polarization of macrophages, and liver metastasis remain unclear. Therefore, it is necessary to explore their influence on the tumor microenvironment of HCC.
Methods
Transmission electron microscopy, nanometer particle testing, and special biomarker analysis were utilized to characterize exosomes, while the differential expression of microRNAs was evaluated using high-throughput sequencing technology. The functions of miR-200b-3p exosomes were confirmed using in vitro and in vivo assays. The interactions between microRNAs and ZEB1 as well as cancer cells and macrophages were measured using RNA pull-down and luciferase gene reporter assays.
Results
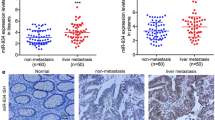
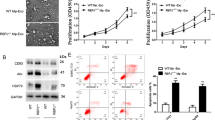
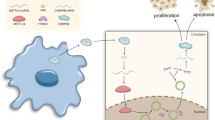
Using in silico analysis, we identified high levels of miR-200b-3p exosome expression in patients with HCC, particularly with relapsed HCC. We demonstrated that HCC cell-derived miR-200b-3p exosomes were internalized by M0 macrophages and induced M2 polarization by downregulating ZEB1 and upregulating interleukin-4. As a result, the JAK/STAT signaling pathway was activated in M2 macrophages, leading to increased PIM1 and VEGFα expression. These cell factors accelerated the proliferation and metastasis of HCC, resulting in a feedback loop between HCC cells and M2 macrophages.
Conclusion
The study illustrates that HCC cell-derived miR-200b-3p exosomes facilitate the proliferation and polarization of macrophages by modulating cytokine secretion and the JAK/STAT signaling pathway, leading to the metastasis of HCC. These findings demonstrate the existence of a novel feedback loop between cancer cells and immune cells in the tumor microenvironment, presenting a new concept in cancer research.









Similar content being viewed by others
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Availability of data and material
All data are available in a public, open-access repository.
Change history
28 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12072-023-10614-w
References
Tran NH. Shifting epidemiology of hepatocellular carcinoma in far Eastern and Southeast Asian patients: explanations and implications. Curr Oncol Rep. 2022;24:187–193
Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell. 2021;39:28–37
Ren X, Zhang L, Zhang Y, Li Z, Siemers N, Zhang Z. Insights gained from single-cell analysis of immune cells in the tumor microenvironment. Annu Rev Immunol. 2021;39:583–609
Ugel S, Cane S, De Sanctis F, Bronte V. Monocytes in the tumor microenvironment. Annu Rev Pathol. 2021;16:93–122
Wong-Rolle A, Wei HK, Zhao C, Jin C. Unexpected guests in the tumor microenvironment: microbiome in cancer. Protein Cell. 2021;12:426–435
Padda J, Khalid K, Khedr A, Patel V, Al-Ewaidat OA, et al. Exosome-derived microRNA: efficacy in cancer. Cureus. 2021;13: e17441
Li C, Qin F, Wang W, Ni Y, Gao M, Guo M, Sun G. hnRNPA2B1-mediated extracellular vesicles sorting of miR-122-5p potentially promotes lung cancer progression. Int J Mol Sci. 2021;22(23):12866
Fu C, Jiang L, Hao S, Liu Z, Ding S, Zhang W, et al. Activation of the IL-4/STAT6 signaling pathway promotes lung cancer progression by increasing M2 myeloid cells. Front Immunol. 2019;10:2638
Lin YJ, Goretzki A, Schulke S. Immune metabolism of IL-4-activated B cells and Th2 cells in the context of allergic diseases. Front Immunol. 2021;12: 790658
Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12:53
Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195
Kugeratski FG, Kalluri R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2021;288:10–35
Matsubara K, Matsubara Y, Uchikura Y, Sugiyama T. Pathophysiology of preeclampsia: the role of exosomes. Int J Mol Sci. 2021;22(5):2572.
Li C, Zhou T, Chen J, Li R, Chen H, Luo S, et al. The role of exosomal miRNAs in cancer. J Transl Med. 2022;20:6
Huang X, Li Y, Fu M, Xin HB. Polarizing macrophages in vitro. Methods Mol Biol. 2018;1784:119–126
Pan J, Zang Y. LINC00667 Promotes progression of esophageal cancer cells by regulating miR-200b-3p/SLC2A3 axis. Dig Dis Sci. 2022;67(7):2936–47.
Ma X, Ying Y, Sun J, Xie H, Li J, He L, et al. circKDM4C enhances bladder cancer invasion and metastasis through miR-200bc-3p/ZEB1 axis. Cell Death Discov. 2021;7:365
Liu L, Jiang H, Pan H, Zhu X. LncRNA XIST promotes liver cancer progression by acting as a molecular sponge of miR-200b-3p to regulate ZEB1/2 expression. J Int Med Res. 2021;49:3000605211016211
Guo Y, Lu X, Chen Y, Rendon B, Mitchell RA, Cuatrecasas M, Cortés M, Postigo A, Liu Y, Dean DC. Zeb1 induces immune checkpoints to form an immunosuppressive envelope around invading cancer cells. Sci Adv. 2021;7(21):eabd7455.
Qian Y, Arellano G, Ifergan I, Lin J, Snowden C, Kim T, et al. ZEB1 promotes pathogenic Th1 and Th17 cell differentiation in multiple sclerosis. Cell Rep. 2021;36: 109602
Plaschka M, Benboubker V, Grimont M, Berthet J, Tonon L, Lopez J, Le-Bouar M, Balme B, Tondeur G, de la Fouchardière A, Larue L, Puisieux A, Grinberg-Bleyer Y, Bendriss-Vermare N, Dubois B, Caux C, Dalle S, Caramel J. ZEB1 transcription factor promotes immune escape in melanoma. J Immunother Cancer. 2022;10(3):e003484
Liu M, Zhang Y, Yang J, Cui X, Zhou Z, Zhan H. ZIP4 increases expression of transcription factor ZEB1 to promote integrin alpha3beta1 signaling and inhibit expression of the gemcitabine transporter ENT1 in pancreatic cancer cells. Gastroenterology. 2020;158:679-692 e671
Feldker N, Ferrazzi F, Schuhwerk H, Widholz SA, Guenther K. Genome-wide cooperation of EMT transcription factor ZEB1 with YAP and AP-1 in breast cancer. EMBO J. 2020;39: e103209
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156
Xun J, Du L, Gao R, Shen L, Wang D, Kang L. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics. 2021;11:6847–6859
Mirlekar B. Tumor promoting roles of IL-10, TGF-beta, IL-4, and IL-35: its implications in cancer immunotherapy. SAGE Open Med. 2022;10:20503121211069012
Chen PH, Anderson L, Zhang K, Weiss GA. Eosinophilic gastritis/gastroenteritis. Curr Gastroenterol Rep. 2021;23:13
Peng M, Zhang Q, Cheng Y, Fu S, Yang H, Guo X, et al. Apolipoprotein A-I mimetic peptide 4F suppresses tumor-associated macrophages and pancreatic cancer progression. Oncotarget. 2017;8:99693–99706
Tao S, Chen Q, Lin C, Dong H. Linc00514 promotes breast cancer metastasis and M2 polarization of tumor-associated macrophages via Jagged1-mediated notch signaling pathway. J Exp Clin Cancer Res. 2020;39:191
Rahal OM, Wolfe AR, Mandal PK, Larson R, Tin S. Blocking interleukin (IL)4- and IL13-mediated phosphorylation of STAT6 (Tyr641) decreases M2 polarization of macrophages and protects against macrophage-mediated radioresistance of inflammatory breast cancer. Int J Radiat Oncol Biol Phys. 2018;100:1034–1043
Cui J, Shan K, Yang Q, Qi Y, Qu H, Li J, et al. Prostaglandin E3 attenuates macrophage-associated inflammation and prostate tumour growth by modulating polarization. J Cell Mol Med. 2021;25:5586–5601
Acknowledgements
Not available.
Funding
Medical and health science and Technology Development of Shandong (202104080599); Natural Science Foundation of Shandong Province (ZR2022QH066).
Author information
Authors and Affiliations
Contributions
YX supervised the study, supported the fund, wrote the manuscript, designed experiments, and constructed figures. GL analyzed some partial data and figures and collected clinical samples. Feng Liu disposed of the review of clinicopathological specimens and guaranteed their source of them. ZL contributed to clinical sample collection and savings. ZL was in charge of in vivo experiments, for example, nude mice were fed and cared for, and subcutaneous tumors were measured. YZ installed and disposed of the data from databases and software. TY managed the color blending of the figures and checked the article format.
Corresponding author
Ethics declarations
Conflict of interest
Ying Xu, Guangchao Luan, Feng Liu, Yuhua Zhang, Zhongchao Li, Ziming Liu and Tao Yang declare that they have no conflicts of interest in this work.
Animal ethics
All animals’ care was in accordance with institution guidelines.
Ethics approval and consent to participate
All procedures involving human participants were performed in accordance with Harbin Medical University’s ethical committee. All patients provided their written informed consent. The study protocol was approved by the institution’s ethics committee research.
Consent for publication
Written informed consent for publication was obtained from all the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Luan, G., Liu, F. et al. Exosomal miR-200b-3p induce macrophage polarization by regulating transcriptional repressor ZEB1 in hepatocellular carcinoma. Hepatol Int 17, 889–903 (2023). https://doi.org/10.1007/s12072-023-10507-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10507-y