Abstract
Background
Fibrosis is the most important pathological feature in predicting development of Hepatocellular carcinoma (HCC). However, the incidence rate of HCC in patients with non-alcoholic fatty liver disease (NAFLD) is relatively low. We evaluated phenotypic histological features to differentiate HCC from non-HCC in patients with non-tumor lesions of cirrhotic livers.
Methods
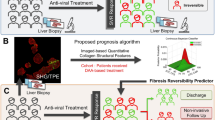
Seventeen patients with NAFLD who underwent liver transplantation were enrolled. FibroNest was used to quantify histological phenotypes of non-tumor fibrosis lesions. Quantification included collagen content and structure traits, fiber morphometric traits, and fibrosis architecture traits. Each trait was described by up to seven quantitative fibrosis traits (qFTs). Among the qFTs measured in each specimen, those that described most of the variability between consecutive groups were automatically detected and combined into a normalized Phenotypic Composite Fibrosis Score (Ph-CFS). We trained FibroNest to identify the principal traits that differentiate HCC from non-HCC.
Results
HCC was found in 8 cases and non-HCC in 9 cases. The Ph-CFS significantly differentiated HCC from non-HCC (4.6 vs. 5.9, p < 0.05). Individual qFTs for morphometric features including collagen fiber length, width, perimeter, and area denoted significant differences between HCC and non-HCC. The Ph-CFS could be used to distinguish HCC (Ph-FCS < 5.0) from non-HCC (Ph-FCS ≥ 5.0) with 75% sensitivity and 100% specificity.
Conclusion
In patients who underwent liver transplantation, fibrotic histological phenotypes in non-tumor lesions appeared to be different between HCC and non-HCC. Phenotypic analysis of collagen in non-tumor lesions might be an effective and automated method to distinguish HCC from non-HCC on histopathology imaging.




Similar content being viewed by others
Data availability
On reasonable request, derived data supporting the findings of this study are available from the corresponding author.
Abbreviations
- LT:
-
Liver transplantation
- qFT:
-
Quantitative fibrosis trait
- Ph-CFS:
-
Phenotypic composite fibrosis score
- HbA1c:
-
Glycated hemoglobin A1c
- BMI:
-
Body mass index
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424
Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(1182–1188): e1181
de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–1200
Sahakyan Y, Lee-Kim V, Bremner KE, Bielecki JM, Krahn MD. Impact of direct-acting antiviral regimens on mortality and morbidity outcomes in patients with chronic hepatitis c: systematic review and meta-analysis. J Viral Hepat. 2021. https://doi.org/10.1111/jvh.13482
Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107
Tateishi R, Uchino K, Fujiwara N, Takehara T, Okanoue T, Seike M, et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J Gastroenterol. 2019;54:367–376
Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650–2666
Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978
Amarapurkar DN, Dharod M, Gautam S, Patel N. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop Gastroenterol. 2013;34:159–163
Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47–53
Kendall TJ, Dolman GE, Duff CM, Paish EC, Zaitoun A, Irving W, et al. Hepatic elastin content is predictive of adverse outcome in advanced fibrotic liver disease. Histopathology. 2018;73:90–100
Yasui Y, Abe T, Kurosaki M, Higuchi M, Komiyama Y, Yoshida T, et al. Elastin fiber accumulation in liver correlates with the development of hepatocellular carcinoma. PLoS ONE. 2016;11: e0154558
Li CI, Chen HJ, Lai HC, Liu CS, Lin WY, Li TC, et al. Hyperglycemia and chronic liver diseases on risk of hepatocellular carcinoma in Chinese patients with type 2 diabetes–National cohort of Taiwan Diabetes Study. Int J Cancer. 2015;136:2668–2679
Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964–973
Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565
Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(389–397): e310
Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res. 2012;347:245–256
Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292
Yoshida K, Matsuzaki K, Murata M, Yamaguchi T, Suwa K, Okazaki K. Clinico-pathological importance of TGF-beta/phospho-smad signaling during human hepatic fibrocarcinogenesis. Cancers (Basel). 2018;10:183
Funding
This work was supported by JSPS KAKENHI Grant Number 21K07916.
Author information
Authors and Affiliations
Contributions
YN and HM were contributed to the conception design of the study. YN and HM wrote original manuscript. YN, HM, SM, YA, RS, MH, MF, AS, MH, SE and KN were contributed collecting data. YN and ON analyzed data. MH, SE, and KN supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors: Yutaka Nakamura, Hisamitsu Miyaaki, Satoshi Miuma, Yuko Akazawa, Masanori Fukusima, Ryu Sasaki, Masafumi Haraguchi, Akihiko Soyama, Masaaki Hidaka, Susumu Eguchi, and Kazuhiko Nakao declare that they have no conflicts to declare.
Animal research (ethics)
It is not applicable.
Consent to participate
Informed consent was obtained in the form of opt-out on the web-site. Those who rejected were excluded.
Consent to publish (ethics)
It is not applicable.
Plant reproducibility
It is not applicable.
Clinical trials registration
It is not applicable.
Ethical approval
This study was conducted in accordance with the 1964 Helsinki declaration and was approved by the Research Ethics Committee of Nagasaki University Hospital (Approval No. 2032316).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12072_2022_10340_MOESM1_ESM.tif
Supplementary file1 Supplemental Figure1; FibroNest™ quantitative digital pathology platform (PharmaNest, Princeton, NJ) can quantify the histological phenotype of fibrosis, including collagen amount and structure (12 traits), the morphometric traits of collagen fibers (13 traits), and architecture of fibrosis (7 traits). Each trait is best described by its statistical distribution across tissues in the form of a histogram. The resulting histogram of each characteristic is described by quantitative fibrosis traits (qFTs) to account for mean, variance, distortion, and progression. Among the 350 qFTs measured in each tissue, the qFTs that describe most of the variability between the groups (principal qFTs). Continuous variables Phenotypic Fibrosis Composite Scores, Phenotypic Heat Chart, and Traits Trajectories can be represented (TIF 354 KB)
Rights and permissions
About this article
Cite this article
Nakamura, Y., Miyaaki, H., Miuma, S. et al. Automated fibrosis phenotyping of liver tissue from non-tumor lesions of patients with and without hepatocellular carcinoma after liver transplantation for non-alcoholic fatty liver disease. Hepatol Int 16, 555–561 (2022). https://doi.org/10.1007/s12072-022-10340-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10340-9