Abstract
Background
Sorafenib is the most widely used first-line treatment for patients with advanced hepatocellular carcinoma (HCC), but such treatment provides only limited survival benefits that might be related to the immune status of distinct tumor microenvironments. A fundamental understanding of the distribution and phenotypes of T lymphocytes in tumors will undoubtedly lead to the development of novel immunotherapeutic strategies that could possibly enhance the efficacy of sorafenib treatments.
Methods
Flow cytometry, immunohistochemistry and immunofluorescence analyses were performed to detect the infiltration and distribution of various leukocyte populations, and the expression of different immune checkpoint molecules in fresh HCC tumor tissues. Correlations among indicating genes were calculated in 365 patients with HCC from The Cancer Genome Atlas (TCGA) data set, and the cumulative overall survival time was calculated using the Kaplan–Meier method. Moreover, role of adenosinergic pathway on sorafenib anti-tumor efficacy was investigated using both subcutaneous and orthotopic transplantation tumor model in immune competent C57BL/6 mice.
Results
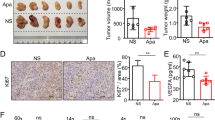
We revealed that levels of CD3+ and CD8+ T cells were significantly downregulated in HCC tumor tissue, so were the infiltration of CD169+ cells (a Mφ subpopulation with T cell activation capacities) and their contact with CD8+ cells in tumor milieus. Moreover, levels of PD-1 and CD39 expression were significantly upregulated in human HCC-infiltrating CD4+ and CD8+ T cells, and CD39+CD8+ T cells exhibited a CD69+PD-1+perforinlowIFNγlow “exhausted” phenotype. Levels of both CD39+ T cells infiltration and adenosine receptor ADORA2B expression in tumor tissues were negatively correlated with overall survival of patients with HCC. Accordingly, mice treated with sorafenib in combination with adenosine A2B receptor blockage reagents exhibited significantly reduced tumor progression compared with control groups.
Conclusions
These results suggest that adenosinergic pathway might represent an applicable target for sorafenib-combined-therapies in human HCC.








Similar content being viewed by others
Data availability
The publicly available microarray data sets analyzed in this study from The Cancer Genome Atlas (TCGA) were downloaded from the data portal of Genomic Data Commons (GDC, https://portal.gdc.cancer.gov/).
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- FDA:
-
Food and Drug Administration
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death ligand 1
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- tSNE:
-
T-distributed stochastic neighbor embedding
- DAPI:
-
Nuclei were stained with 4′,6-diamidino-2-phenylindole
- BFA:
-
Brefeldin A
- TCGA:
-
The Cancer Genome Atlas
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Dawkins J, Webster RM. The hepatocellular carcinoma market. Nat Rev Drug Discov. 2019;18(1):13–4.
Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–35.
Buonaguro L, Mauriello A, Cavalluzzo B, Petrizzo A, Tagliamonte M. Immunotherapy in hepatocellular carcinoma. Ann Hepatol. 2019;18(2):291–7.
Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13(2):123–35.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–43.
Chen ML, Yan BS, Lu WC, Chen MH, Yu SL, Yang PC, et al. Sorafenib relieves cell-intrinsic and cell-extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. Int J Cancer. 2014;134(2):319–31.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22.
He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–60.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26.
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502.
Siu LL, Ivy SP, Dixon EL, Gravell AE, Reeves SA, Rosner GL. Challenges and opportunities in adapting clinical trial design for immunotherapies. Clin Cancer Res. 2017;23(17):4950–8.
Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):709–24.
Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene. 2017;36(3):293–303.
Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276(1):121–44.
Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019;27(8):2411–2425.e9.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9.
Chu Y, Liao J, Li J, Wang Y, Yu J, Wang J, et al. CD103+ tumor-infiltrating lymphocytes predict favorable prognosis in patients with esophageal squamous cell carcinoma. J Cancer. 2019;10(21):5234–43.
Zhang Y, Li JQ, Jiang ZZ, Li L, Wu Y, Zheng L. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J Pathol. 2016;239(2):231–41.
Asano K, Nabeyama A, Miyake Y, Qiu CH, Kurita A, Tomura M, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34(1):85–95.
Martinez-Pomares L, Gordon S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol. 2012;33(2):66–70.
Fraschilla I, Pillai S. Viewing Siglecs through the lens of tumor immunology. Immunol Rev. 2017;276(1):178–91.
Noble A, Mehta H, Lovell A, Papaioannou E, Fairbanks L. IL-12 and IL-4 activate a CD39-dependent intrinsic peripheral tolerance mechanism in CD8(+) T cells. Eur J Immunol. 2016;46(6):1438–48.
Gouttefangeas C, Mansur I, Schmid M, Dastot H, Gelin C, Mahouy G, et al. The CD39 molecule defines distinct cytotoxic subsets within alloactivated human CD8-positive cells. Eur J Immunol. 1992;22(10):2681–5.
Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–9.
Canale FP, Ramello MC, Nunez N, Araujo Furlan CL, Bossio SN, Gorosito Serran M, et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8(+) T cells. Cancer Res. 2018;78(1):115–28.
Raoul JL, Kudo M, Finn RS, Edeline J, Reig M, Galle PR. Systemic therapy for intermediate and advanced hepatocellular carcinoma: sorafenib and beyond. Cancer Treat Rev. 2018;68:16–24.
Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15(10):599–616.
Fu YP, Yi Y, Cai XY, Sun J, Ni XC, He HW, et al. Overexpression of interleukin-35 associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Br J Cancer. 2016;114(7):767–76.
Ma XL, Shen MN, Hu B, Wang BL, Yang WJ, Lv LH, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110beta and predicts poor prognosis. J Hematol Oncol. 2019;12(1):37.
Liao J, Luan Y, Ren Z, Liu X, Xue D, Xu H, et al. Converting lymphoma cells into potent antigen-presenting cells for interferon-induced tumor regression. Cancer Immunol Res. 2017;5(7):560–70.
Sun C, Xu J, Song J, Liu C, Wang J, Weng C, et al. The predictive value of centre tumour CD8(+) T cells in patients with hepatocellular carcinoma: comparison with immunoscore. Oncotarget. 2015;6(34):35602–15.
Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25(2):214–21.
Xu MM, Pu Y, Zhang Y, Fu YX. The role of adaptive immunity in the efficacy of targeted cancer therapies. Trends Immunol. 2016;37(2):141–53.
Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37(1):110.
Takenaka MC, Robson S, Quintana FJ. Regulation of the T cell response by CD39. Trends Immunol. 2016;37(7):427–39.
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–65.
Bloy N, Pol J, Manic G, Vitale I, Eggermont A, Galon J, et al. Trial watch: radioimmunotherapy for oncological indications. Oncoimmunology. 2014;3(9):e954929.
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14.
Seebacher NA, Stacy AE, Porter GM, Merlot AM. Clinical development of targeted and immune based anti-cancer therapies. J Exp Clin Cancer Res. 2019;38(1):156.
Xu W, Yang Z, Lu N. Molecular targeted therapy for the treatment of gastric cancer. J Exp Clin Cancer Res. 2016;35:1.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50.
Tang H, Fu YX. Immune evasion in tumor’s own sweet way. Cell Metab. 2018;27(5):945–6.
Wang L, Wang FS. Clinical immunology and immunotherapy for hepatocellular carcinoma: current progress and challenges. Hepatol Int. 2019;13(5):521–33.
Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13(2):125–37.
Nishida N, Kudo M. Liver damage related to immune checkpoint inhibitors. Hepatol Int. 2019;13(3):248–52.
Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18(12):1332–41.
Funding
This work was supported by project grants from the National Natural Science Foundation of China (81773054 to Y.W., and 31900651 to J.L.), the National Key Research and Development Plan of China (2018ZX10302205 to L.Z.), the China Postdoctoral Science Foundation (2018M643302 to J.L.), and the Fundamental Research Funds for the Central Universities (171gjc32 to L.Z.).
Author information
Authors and Affiliations
Contributions
JL, YW, and LZ designed the experiments and analyzed the data. JL, DNZ, JZL, QMH, LYZ and YC performed the experiments. ZX, CH, KM, and WPW provided the reagents. JL and YW wrote the manuscript. YW and LZ supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All samples were anonymously coded in accord with local ethical guidelines (as stipulated by the Declaration of Helsinki) and with written informed consent. The Review Board of Sun Yat-sen University Cancer Center approved the study protocol. All animal procedures were performed in accordance with the experimental animal guidelines set by the Institutional Animal Care and Use Committee of Sun Yat-sen University Cancer Center (L102012018120M).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liao, J., Zeng, DN., Li, JZ. et al. Targeting adenosinergic pathway enhances the anti-tumor efficacy of sorafenib in hepatocellular carcinoma. Hepatol Int 14, 80–95 (2020). https://doi.org/10.1007/s12072-019-10003-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-10003-2