Abstract
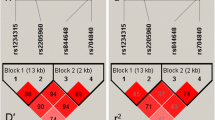
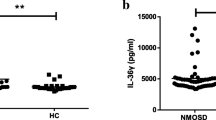
Interferon regulatory factor 5 (IRF5) is a critical transcription factor in the toll-like receptor signaling pathway. It is associated with autoimmune disorders, such as rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease. However, the relationship between the functional single nucleotide polymorphisms (SNPs) of IRF5 and its mRNA expression level in patients with neuromyelitis optica spectrum disorder remains unclear. The present study aimed to investigate the relationship between polymorphisms and mRNA expression levels of the IRF5 gene with the incidence of neuromyelitis optica spectrum disorder (NMOSD) in northern Chinese Han people. Two loci of the IRF5 gene (rs2004640 and rs2280714) of 164 patients with NMOSD and 269 healthy subjects were genotyped using the multiple SNaPshot technique. The frequencies of alleles, genotypes, and haplotypes were compared. Stratified analysis was performed according to age, sex, AQP4 status, onset age, and Expanded Disability Status Scale (EDSS) score. The IRF5 mRNA levels in peripheral blood mononuclear cells (PBMCs) of 64 NMOSD patients (32 patients in the acute stage and 32 patients in the remission stage) and 35 healthy subjects were detected by real-time PCR. The association of SNP polymorphisms with the mRNA expression level was determined by nonparametric tests. Allele and genotype frequency distributions of rs2004640 showed significant differences between both groups. Compared to healthy controls, the frequency of rs2004640 T allele markedly increased in patients (OR = 1.51, 95% CI = 1.09–2.08, P = 0.005). Minor allele T and GT genotype of rs2004640 that significantly increases the risk of NMOSD were discovered using genetic inheritance models (codominant, dominant, and overdominant) and haplotype analyses. Subsequent haplotype analyses revealed that the major haplotype “T-A” containing the risk alleles (the SNP sequence of the alleles was rs2004640 and rs2280714) had adverse effects on NMOSD. Based on the stratification analysis according to the EDSS score, the GT genotype frequency in the EDSS ≥ 4 group (38.2%) was markedly lower than that in the EDSS < 4 group (61.8%) (OR = 0.32, 95% CI = 0.15–0.68, P = 0.0054), with a significant difference. The IRF5 mRNA expression level was increased in NMOSD patients compared to that in normal subjects. IRF5 gene polymorphisms may be tightly associated with the genesis and progression of NMOSD in northern Chinese Han people. IRF5 mRNA expression was increased in patients with NMOSD and significantly increased in patients with acute phase. Perhaps IRF5 expression levels can be used as a predictor of disease activity in the future.
Highlights
IRF5 gene (rs2004640) shows significant differences between both NMOSD and healthy controls.
Minor allele T and GT genotype of rs2004640 significantly increase the risk of NMOSD.
IRF5 mRNA expression level increases in NMOSD patients compared to normal subjects.
The rs2004640 polymorphism of the IRF5 gene is highly correlated with susceptibility to NMOSD.
Significance Statement
NMOSD is a relatively rare inflammatory, autoimmune, and demyelinating disorder of the CNS, which predominantly affects the optic nerve and spinal cord. IRF5 gene plays a crucial role in regulating immune responses and has been implicated in various autoimmune diseases. This study investigated the association of IRF5 gene polymorphisms and mRNA expression levels with NMOSD. The findings reveal a significant correlation between specific IRF5 gene polymorphisms and increased susceptibility to NMOSD. To the best of our knowledge, the association of IRF5 gene polymorphisms and mRNA expression levels with NMOSD has not been investigated. Our study provides a genetic perspective on the importance of IRF5 in NMOSD pathogenesis and promotes the development of screening strategies that target the alleles susceptible to NMOSD for early control.



Similar content being viewed by others
Data Availability
All processed data used in this study can be obtained from the corresponding author upon reasonable request.
References
Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B (2020) Neuromyelitis Optica. Nat Reviews Disease Primers 6(1):85. https://doi.org/10.1038/s41572-020-0214-9
Jarius S, Wildemann B (2013) The history of neuromyelitis optica. J Neuroinflamm 10:8. https://doi.org/10.1186/1742-2094-10-8
Jarius S, Wildemann B (2019) Devic’s index case: a critical reappraisal - AQP4-IgG-mediated neuromyelitis optica spectrum disorder, or rather MOG encephalomyelitis? J Neurol Sci 407:116396. https://doi.org/10.1016/j.jns.2019.07.014
Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG (2007) The spectrum of neuromyelitis optica. Lancet Neurol 6(9):805–815. https://doi.org/10.1016/s1474-4422(07)70216-8
Bennett JL, O’Connor KC, Bar-Or A, Zamvil SS, Hemmer B, Tedder TF, von Büdingen HC, Stuve O, Yeaman MR, Smith TJ, Stadelmann C (2015) B lymphocytes in neuromyelitis optica. Neurology(R) neuroimmunology & neuroinflammation 2 (3):e104. https://doi.org/10.1212/nxi.0000000000000104
Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D (2012) B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209(5):1001–1010. https://doi.org/10.1084/jem.20111675
Kalampokis I, Yoshizaki A, Tedder TF (2013) IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Therapy 15(S1):1–12. https://doi.org/10.1186/ar3907
Matiello M, Kim HJ, Kim W, Brum DG, Barreira AA, Kingsbury DJ, Plant GT, Adoni T, Weinshenker BG (2010) Familial neuromyelitis optica. Neurology 75(4):310–315. https://doi.org/10.1212/WNL.0b013e3181ea9f15
Yamakawa K, Kuroda H, Fujihara K, Sato S, Nakashima I, Takeda A, Suzuki K, Itoyama Y (2000) Familial neuromyelitis optica (Devic’s syndrome) with late onset in Japan. Neurology 55(2):318–320. https://doi.org/10.1212/wnl.55.2.318
Bruijstens AL, Wong YYM, van Pelt DE, van der Linden PJE, Haasnoot GW, Hintzen RQ, Claas FHJ, Neuteboom RF, Wokke BHA (2020) HLA association in MOG-IgG- and AQP4-IgG-related disorders of the CNS in the Dutch population. Neurology(R) Neuroimmunol Neuroinflammation 7(3). https://doi.org/10.1212/nxi.0000000000000702
Alonso VR, de Jesus Flores Rivera J, Garci YR, Granados J, Sanchez T, Mena-Hernandez L, Corona T (2018) Neuromyelitis Optica (NMO IgG+) and genetic susceptibility, potential ethnic influences. Cent Nerv Syst Agents Med Chem 18(1):4–7. https://doi.org/10.2174/1871524916666160229115047
Pandit L, Malli C, D’Cunha A, Mustafa S (2015) Human leukocyte antigen association with neuromyelitis optica in a south Indian population. Mult Scler 21(9):1217–1218. https://doi.org/10.1177/1352458515574149
Ogawa K, Okuno T, Hosomichi K, Hosokawa A, Hirata J, Suzuki K, Sakaue S, Kinoshita M, Asano Y, Miyamoto K, Inoue I, Kusunoki S, Okada Y, Mochizuki H (2019) Next-generation sequencing identifies contribution of both class I and II HLA genes on susceptibility of multiple sclerosis in Japanese. J Neuroinflamm 16(1):162. https://doi.org/10.1186/s12974-019-1551-z
Wang H, Dai Y, Qiu W, Zhong X, Wu A, Wang Y, Lu Z, Bao J, Hu X (2011) HLA-DPB1 0501 is associated with susceptibility to anti-aquaporin-4 antibodies positive neuromyelitis optica in southern Han Chinese. J Neuroimmunol 233(1–2):181–184. https://doi.org/10.1016/j.jneuroim.2010.11.004
Negishi H, Taniguchi T, Yanai H (2018) The Interferon (IFN) Class of cytokines and the IFN Regulatory Factor (IRF) transcription factor family. Cold Spring Harb Perspect Biol 10(11). https://doi.org/10.1101/cshperspect.a028423
Sweeney SE (2011) Targeting interferon regulatory factors to inhibit activation of the type I IFN response: implications for treatment of autoimmune disorders. Cell Immunol 271(2):342–349. https://doi.org/10.1016/j.cellimm.2011.07.014
Feng D, Sangster-Guity N, Stone R, Korczeniewska J, Mancl ME, Fitzgerald-Bocarsly P, Barnes BJ (2010) Differential requirement of histone acetylase and deacetylase activities for IRF5-mediated proinflammatory cytokine expression. J Immunol 185(10):6003–6012. https://doi.org/10.4049/jimmunol.1000482
Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, Taniguchi T (2005) Integral role of IRF-5 in the gene induction programme activated by toll-like receptors. Nature 434(7030):243–249. https://doi.org/10.1038/nature03308
Tamura T, Yanai H, Savitsky D, Taniguchi T (2008) The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 26:535–584. https://doi.org/10.1146/annurev.immunol.26.021607.090400
Graham RR, Kozyrev SV, Baechler EC, Reddy MV, Plenge RM, Bauer JW, Ortmann WA, Koeuth T, González Escribano MF, Pons-Estel B, Petri M, Daly M, Gregersen PK, Martín J, Altshuler D, Behrens TW, Alarcón-Riquelme ME (2006) A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet 38(5):550–555. https://doi.org/10.1038/ng1782
Sigurdsson S, Nordmark G, Göring HH, Lindroos K, Wiman AC, Sturfelt G, Jönsen A, Rantapää-Dahlqvist S, Möller B, Kere J, Koskenmies S, Widén E, Eloranta ML, Julkunen H, Kristjansdottir H, Steinsson K, Alm G, Rönnblom L, Syvänen AC (2005) Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet 76(3):528–537. https://doi.org/10.1086/428480
Tan CT, Mao Z, Qiu W, Hu X, Wingerchuk DM, Weinshenker BG (2016) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 86(5):491–492. https://doi.org/10.1212/wnl.0000000000002366
Sicras-Mainar A, Ruíz-Beato E, Navarro-Artieda R, Maurino J (2017) Impact on healthcare resource utilization of multiple sclerosis in Spain. BMC Health Serv Res 17(1):854. https://doi.org/10.1186/s12913-017-2807-x
Shi YY, He L (2023) Publisher correction: SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. https://doi.org/10.1038/s41422-023-00805-3
Mehta B, Daniel R, Phillips C, McNevin D (2017) Forensically relevant SNaPshot(®) assays for human DNA SNP analysis: a review. Int J Legal Med 131(1):21–37. https://doi.org/10.1007/s00414-016-1490-5
Honda K, Taniguchi T (2006) IRFs: master regulators of signalling by toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 6(9):644–658. https://doi.org/10.1038/nri1900
Tang L, Chen B, Ma B, Nie S (2014) Association between IRF5 polymorphisms and autoimmune diseases: a meta-analysis. Genet Mol Research: GMR 13(2):4473–4485. https://doi.org/10.4238/2014.June.16.6
Tang L, Wan P, Wang Y, Pan J, Wang Y, Chen B (2015) Genetic association and interaction between the IRF5 and TYK2 genes and systemic lupus erythematosus in the Han Chinese population. Inflamm Research: Official J Eur Histamine Res Soc [et al] 64(10):817–824. https://doi.org/10.1007/s00011-015-0865-2
Sigurdsson S, Göring HH, Kristjansdottir G, Milani L, Nordmark G, Sandling JK, Eloranta ML, Feng D, Sangster-Guity N, Gunnarsson I, Svenungsson E, Sturfelt G, Jönsen A, Truedsson L, Barnes BJ, Alm G, Rönnblom L, Syvänen AC (2008) Comprehensive evaluation of the genetic variants of interferon regulatory factor 5 (IRF5) reveals a novel 5 bp length polymorphism as strong risk factor for systemic lupus erythematosus. Hum Mol Genet 17(6):872–881. https://doi.org/10.1093/hmg/ddm359
Hu W, Ren H (2011) A meta-analysis of the association of IRF5 polymorphism with systemic lupus erythematosus. Int J Immunogenet 38(5):411–417. https://doi.org/10.1111/j.1744-313X.2011.01025.x
Niewold TB, Kelly JA, Kariuki SN, Franek BS, Kumar AA, Kaufman KM, Thomas K, Walker D, Kamp S, Frost JM, Wong AK, Merrill JT, Alarcón-Riquelme ME, Tikly M, Ramsey-Goldman R, Reveille JD, Petri MA, Edberg JC, Kimberly RP, Alarcón GS, Kamen DL, Gilkeson GS, Vyse TJ, James JA, Gaffney PM, Moser KL, Crow MK, Harley JB (2012) IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann Rheum Dis 71(3):463–468. https://doi.org/10.1136/annrheumdis-2011-200463
Kristjansdottir G, Sandling JK, Bonetti A, Roos IM, Milani L, Wang C, Gustafsdottir SM, Sigurdsson S, Lundmark A, Tienari PJ, Koivisto K, Elovaara I, Pirttilä T, Reunanen M, Peltonen L, Saarela J, Hillert J, Olsson T, Landegren U, Alcina A, Fernández O, Leyva L, Guerrero M, Lucas M, Izquierdo G, Matesanz F, Syvänen AC (2008) Interferon regulatory factor 5 (IRF5) gene variants are associated with multiple sclerosis in three distinct populations. J Med Genet 45(6):362–369. https://doi.org/10.1136/jmg.2007.055012
Liu QB, Wu L, Zhao GX, Cai PP, Li ZX, Wu ZY (2015) Variants of interferon regulatory factor 5 are associated with neither neuromyelitis optica nor multiple sclerosis in the southeastern Han Chinese population. Chin Med J 128(13):1743–1747. https://doi.org/10.4103/0366-6999.159347
Yin BW, Li B, Mehmood A, Yuan C, Song S, Guo RY, Zhang L, Ma T, Guo L (2021) BLK polymorphisms and expression level in neuromyelitis optica spectrum disorder. CNS Neurosci Ther 27(12):1549–1560. https://doi.org/10.1111/cns.13738
Acknowledgements
The authors are grateful to the NMOSD patients and healthy volunteers for participating in this study. Also, we would like to thank Mr. Arshad Mehmood for assisting with professional editing and proofreading for this manuscript.
Funding
This study was supported by the Hebei Natural Science Foundation (No. H2022206483) and the S&T Program of Hebei (No. 22377711D).
Author information
Authors and Affiliations
Contributions
Gaoning Wang: Conceptualization. Liu Jing and Ying Wang: Data curation. Arshad Mehmood: Writing-review & editing. Huining Zhang: Validation. Ruoyi Guo and Lu Zhang: Software. Bin Li: Supervision. All the authors have approved the submission.
Corresponding author
Ethics declarations
Ethical Approval
The experiments were performed at the Key Laboratory of Neurology of Hebei Province and were approved by the Experimental Ethics Committee of the Second Hospital of Hebei Medical University.
Consent to Participate
Before this study, all participants gave informed written consent.
Consent to Publish
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Jing, L., Wang, Y. et al. Interferon Regulatory Factor 5 Gene Polymorphisms and mRNA Expression Levels Are Associated with Neuromyelitis Optica Spectrum Disorder. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04072-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04072-0