Abstract
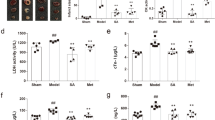
As a famous prescription in China, AnGong NiuHuang (AGNH) pill exerts good neuroprotection for ischaemic stroke (IS), but its mechanism is still unclear. In this study, the neuroprotection of AGNH was evaluated in the rat IS model which were established with the surgery of middle cerebral artery occlusion (MCAO), and the potential mechanism was elucidated by transcriptomic analysis and metabolomic analysis. AGNH treatment obviously decreased the infarct volume and Zea-Longa 5-point neurological deficit scores, improved the survival percentage of rats, regional cerebral blood flow (rCBF), and rat activity distance and activity time. Transcriptomics showed that AGNH exerted its anti-inflammatory effects by affecting the regulatory network including Tyrobp, Syk, Tlr2, Myd88 and Ccl2 as the core. Integrating transcriptomics and metabolomics identified 8 key metabolites regulated by AGNH, including L-histidine, L-serine, L-alanine, fumaric acid, malic acid, and N-(L-arginino) succinate, 1-pyrroline-4-hydroxy-2-carboxylate and 1-methylhistamine in the rats with IS. Additionally, AGNH obviously reduced Tyrobp, Syk, Tlr2, Myd88 and Ccl2 at both the mRNA and protein levels, decreased IL-1β, KC-GRO, IL-13, TNF-α, cleaved caspase 3 and p65 nucleus translocation, but increased IκBα expression. Network pharmacology analysis showed that quercetin, beta-sitosterol, baicalein, naringenin, acacetin, berberine and palmatine may play an important role in protecting against IS. Taken together, this study reveals that AGNH reduced neuroinflammation and protected against IS by inhibiting Tyrobp/Syk and Tlr2/Myd88, as well as NF-κB signalling pathway and regulating multiple metabolites.








Similar content being viewed by others
Data Availability
All data supporting described findings can be obtained from the corresponding authors upon reasonable request.
Abbreviations
- IS:
-
Ischaemic stroke
- AD:
-
Alzheimer disease
- AGNH:
-
AnGong NiuHuang
- BBB:
-
Blood-brain barrier
- DEGs:
-
Differentially expressed genes
- DMs:
-
Differentially expressed metabolites
- ELISA:
-
Enzyme-linked immunosorbent assay
- GO:
-
Gene Ontology
- rCBF:
-
Regional cerebral blood flow
- I/R:
-
Ischaemia/reperfusion
- IF:
-
Immunofluorescence staining
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MCAO:
-
Middle cerebral artery occlusion
- SD:
-
Sprague-Dawley
- TCM:
-
Traditional Chinese medicine
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- Ccl2:
-
C-C motif chemokine ligand 2
- Tlr2:
-
Toll-like receptor 2
- Tlr7:
-
Toll-like receptor 7
- Myd88:
-
Myeloid differentiation factor 88
- Tyrobp:
-
TYRO protein tyrosine kinase-binding protein
- Syk:
-
Spleen tyrosine kinase
References
Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA et al (2014) Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 383(9913):245–254. https://doi.org/10.1016/S0140-6736(13)61953-4
Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C (2012) Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci 13(9):11753–11772. https://doi.org/10.3390/ijms130911753
Shi H, Liu KJ (2007) Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front Biosci 12:1318–1328. https://doi.org/10.2741/2150
Chamorro A, Dirnagl U, Urra X, Planas AM (2016) Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 15(8):869–881. https://doi.org/10.1016/S1474-4422(16)00114-9
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD (2019) Global brain inflammation in stroke. Lancet Neurol 18(11):1058–1066. https://doi.org/10.1016/S1474-4422(19)30078-X
Hou J, Cao G, Tian L, Zhou R, Zhang Y, Xu H, Wu HW, Wang LF, Yang HJ, Zhang JJ (2022) Integrated transcriptomics and metabolomics analysis reveals that C3 and C5 are vital targets of DuZhi Wan in protecting against cerebral ischemic injury. Biomed Pharmacother 155:113703. https://doi.org/10.1016/j.biopha.2022.113703
Sun K, Fan J, Han J (2015) Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B 5(1):8–24. https://doi.org/10.1016/j.apsb.2014.11.002
Zhang J, Zhou R, Xiang C, Fan F, Gao J, Zhang Y et al (2020) Enhanced thioredoxin, glutathione and Nrf2 antioxidant systems by safflower extract and aceglutamide attenuate cerebral ischaemia/reperfusion injury. J Cell Mol Med 24(9):4967–4980. https://doi.org/10.1111/jcmm.15099
Zhang J, Zhou R, Cao G, Zhang Y, Xu H, Yang H (2022) Guhong injection prevents ischemic stroke-induced neuro-inflammation and neuron loss through regulation of C5ar1. Front Pharmacol 13:818245. https://doi.org/10.3389/fphar.2022.818245
Yang X, Wang L, Li J, Liang N, Wang Y, Liu J (2018) Chinese herbal medicine Dengzhan Shengmai capsule as adjunctive treatment for ischemic stroke: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med 36:82–89. https://doi.org/10.1016/j.ctim.2017.12.004
Cai Y, Zhang X, Huang Y, Wang L, Sun J, Liang W et al (2019) The add-on effect of dengzhan shengmai capsules on secondary prevention of ischemic stroke: a multicentre, randomised, placebo-controlled clinical trial. Complement Ther Med 46:189–194. https://doi.org/10.1016/j.ctim.2019.08.015
Commission CP (2015) Pharmacopoeia of the People’s Republic of China p.879. Chinese Medical Science and Technology Press, Beijing
Bai X, GFF, Tong L, Yang HJ (2023) CiteSpace knowledge map analysis of Angong Niuhuang pills in recent 20 years. Zhongguo Zhong Yao Za Zhi, 48(5). https://doi.org/10.19540/j.cnki.cjcmm.20221025.501
Guo Y, Yan S, Xu L, Zhu G, Yu X, Tong X (2014) Use of angong niuhuang in treating central nervous system diseases and related research. Evid Based Complement Alternat Med 2014:346918. https://doi.org/10.1155/2014/346918
Liu H, Yan Y, Pang P, Mao J, Hu X, Li D, Zhou B, Shan H (2019) Angong Niuhuang pill as adjuvant therapy for treating acute cerebral infarction and intracerebral hemorrhage: a meta-analysis of randomized controlled trials. J Ethnopharmacol 237:307–313. https://doi.org/10.1016/j.jep.2019.03.043
Tsoi B, Chen X, Gao C, Wang S, Yuen SC, Yang D et al (2019) Neuroprotective effects and hepatorenal toxicity of Angong Niuhuang Wan against ischemia-reperfusion brain injury in rats. Front Pharmacol 10:593. https://doi.org/10.3389/fphar.2019.00593
Chen H, Luo Y, Tsoi B, Gu B, Qi S, Shen J (2022) Angong Niuhuang Wan reduces hemorrhagic transformation and mortality in ischemic stroke rats with delayed thrombolysis: involvement of peroxynitrite-mediated MMP-9 activation. Chin Med 17(1):51. https://doi.org/10.1186/s13020-022-00595-7
Zhang H, Hui X, Wang Y, Wang Y, Lu X (2022) Angong Niuhuang pill ameliorates cerebral ischemia/reperfusion injury in mice partly by restoring gut microbiota dysbiosis. Front Pharmacol 13:1001422. https://doi.org/10.3389/fphar.2022.1001422
Zhang J, Zhou R, Deng L, Cao G, Zhang Y, Xu H, Hou JY, Ju S, Yang HJ (2022) Huangbai liniment and berberine promoted wound healing in high-fat diet/streptozotocin-induced diabetic rats. Biomed Pharmacother 150:112948. https://doi.org/10.1016/j.biopha.2022.112948
Li X, Zhang D, Bai Y, Xiao J, Jiao H, He R (2019) Ginaton improves neurological function in ischemic stroke rats via inducing autophagy and maintaining mitochondrial homeostasis. Neuropsychiatr Dis Treat 15:1813–1822. https://doi.org/10.2147/NDT.S205612
Zhang J, Guo F, Zhou R, Xiang C, Zhang Y, Gao J et al (2021) Proteomics and transcriptome reveal the key transcription factors mediating the protection of Panax notoginseng saponins (PNS) against cerebral ischemia/reperfusion injury. Phytomedicine 92:153613. https://doi.org/10.1016/j.phymed.2021.153613
Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S (2004) Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol 56(4):468–477. https://doi.org/10.1002/ana.20199
Candelario-Jalil E, Yang Y, Rosenberg GA (2009) Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 158(3):983–994. https://doi.org/10.1016/j.neuroscience.2008.06.025
Neumann J, Riek-Burchardt M, Herz J, Doeppner TR, Konig R, Hutten H et al (2015) Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol 129(2):259–277. https://doi.org/10.1007/s00401-014-1355-2
Haure-Mirande JV, Audrain M, Ehrlich ME, Gandy S (2022) Microglial TYROBP/DAP12 in Alzheimer’s disease: transduction of physiological and pathological signals across TREM2. Mol Neurodegener 17(1):55. https://doi.org/10.1186/s13024-022-00552-w
Hickman S, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK et al (2013) The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16(12):1896–1905. https://doi.org/10.1038/nn.3554
Audrain M, Haure-Mirande JV, Mleczko J, Wang M, Griffin JK, St George-Hyslop PH et al (2021) Reactive or transgenic increase in microglial TYROBP reveals a TREM2-independent TYROBP-APOE link in wild-type and Alzheimer’s-related mice. Alzheimers Dement 17(2):149–163. https://doi.org/10.1002/alz.12256
Li R, Lv ZY, Li YX, Li W, Hao YL (2022) Effects of TYROBP deficiency on neuroinflammation of a Alzheimer’s disease mouse model carrying a PSEN1 p.G378E mutation. Chin Med Sci J 37(4):320–330. https://doi.org/10.24920/004059
Yasukawa S, Miyazaki Y, Yoshii C, Nakaya M, Ozaki N, Toda S et al (2014) An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nat Commun 5:3755. https://doi.org/10.1038/ncomms4755
Ye XC, Hao Q, Ma WJ, Zhao QC, Wang WW, Yin HH et al (2020) Dectin-1/Syk signaling triggers neuroinflammation after ischemic stroke in mice. J Neuroinflammation 17(1):17. https://doi.org/10.1186/s12974-019-1693-z
van Eeuwijk JM, Stegner D, Lamb DJ, Kraft P, Beck S, Thielmann I, Kiefer F, Walzog B, Stoll G, Nieswandt B (2016) The novel oral syk inhibitor, Bl1002494, protects mice from arterial thrombosis and thromboinflammatory brain infarction. Arterioscler Thromb Vasc Biol 36(6):1247–1253. https://doi.org/10.1161/ATVBAHA.115.306883
Tajalli-Nezhad, S, Karimian, M, Beyer, C, Atlasi MA, Azami Tameh, A (2019) The regulatory role of Toll-like receptors after ischemic stroke: neurosteroids as TLR modulators with the focus on TLR2/4. Cell Mol Life Sci, 76(3): p. 523–537. https://doi.org/10.1007/s00018-018-2953-2
Ziegler G, Freyer D, Harhausen D, Khojasteh U, Nietfeld W, Trendelenburg G (2011) Blocking TLR2 in vivo protects against accumulation of inflammatory cells and neuronal injury in experimental stroke. J Cereb Blood Flow Metab 31(2):757–766. https://doi.org/10.1038/jcbfm.2010.161
Li L, Lou W, Li H, Zhu Y, Huang X (2020) Upregulated C-C motif chemokine ligand 2 promotes ischemic stroke via chemokine signaling pathway. Ann Vasc Surg 68:476–486. https://doi.org/10.1016/j.avsg.2020.04.047
Vera-Aviles M, Vantana E, Kardinasari E, Koh NL, Latunde-Dada GO (2018) Protective role of histidine supplementation against oxidative stress damage in the management of anemia of chronic kidney disease. Pharmaceuticals (Basel) 11(4). https://doi.org/10.3390/ph11040111
Sánchez-Pérez S, Celorio-Sardà R, Veciana-Nogués MT, Latorre-Moratalla ML, Comas-Basté O, Vidal-Carou MC (2022) 1-Methylhistamine as a potential biomarker of food histamine intolerance. A pilot study. Front Nutr 9:973682. https://doi.org/10.3389/fnut.2022.973682
He, F, Yin, Z, Wu, C, Xia, Y, Wu, M, Li, , et.al. (2019) l-Serine lowers the inflammatory responses during Pasteurella multocida infection. Infect Immun, 87(12). https://doi.org/10.1128/iai.00677-19
Zhai P, Xu L, Yang J, Jiang Z, Zhao G, Sun L et al (2015) Reduction of inflammatory responses by L-serine treatment leads to neuroprotection in mice after traumatic brain injury. Neuropharmacology 95:1–11. https://doi.org/10.1016/j.neuropharm.2015.02.026
Cunningham GA, McClenaghan NH, Flatt PR, Newsholme P (2005) L-alanine induces changes in metabolic and signal transduction gene expression in a clonal rat pancreatic beta-cell line and protects from pro-inflammatory cytokine-induced apoptosis. Clin Sci (Lond) 109(5):447–455. https://doi.org/10.1042/CS20050149
Cordaro M, Casili G, Paterniti I, Cuzzocrea S, Esposito E (2017) Fumaric acid esters attenuate secondary degeneration after spinal cord injury. J Neurotrauma 34(21):3027–3040. https://doi.org/10.1089/neu.2016.4678
Gold R, Linker RA, Stangel M (2012) Fumaric acid and its esters: an emerging treatment for multiple sclerosis with antioxidative mechanism of action. Clin Immunol 142(1):44–48. https://doi.org/10.1016/j.clim.2011.02.017
Tang X, Liu J, Dong W, Li P, Li L, Lin C, Zheng Y, Hou J, Li D (2013) The cardioprotective effects of citric acid and L-malic acid on myocardial ischemia/reperfusion injury. Evid Based Complement Alternat Med 2013:820695. https://doi.org/10.1155/2013/820695
Chen SF, PM, Tang JC, Cheng J, Zhao D, Zhang Y, Liao HB, Liu R, Zhuang Y, Zhang ZF, Chen J, Lei RX, Li SF, Li HT, Wang ZF, Wan Q (2020) Arginine is neuroprotective through suppressing HIF-1α/LDHA-mediated inflammatory response after cerebral ischemia/reperfusion injury. Mol Brain, 13(1): p. 63. https://doi.org/10.1186/s13041-020-00601-9
Zhang L et al (2022) Neuroprotective effects of quercetin on ischemic stroke: a literature review. Front Pharmacol 13:854249. https://doi.org/10.3389/fphar.2022.854249
Cen J et al (2022) A water-soluble quercetin conjugate with triple targeting exerts neuron-protective effect on cerebral ischemia by mitophagy activation. Adv Healthc Mater 11(22):e2200817. https://doi.org/10.1002/adhm.202200817
Tang X et al (2024) Treatment with beta-sitosterol ameliorates the effects of cerebral ischemia/reperfusion injury by suppressing cholesterol overload, endoplasmic reticulum stress, and apoptosis. Neural Regen Res 19(3):642–649. https://doi.org/10.4103/1673-5374.380904
Li M et al (2022) Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem Biol Interact 366:110137. https://doi.org/10.1016/j.cbi.2022.110137
Bai X et al (2014) Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-kappaB, MMP-9 and up-regulated claudin-5 expression. Neurochem Res 39(8):1405–1415. https://doi.org/10.1007/s11064-014-1326-y
Bu J et al (2019) Acacetin protects against cerebral ischemia-reperfusion injury via the NLRP3 signaling pathway. Neural Regen Res 14(4):605–612. https://doi.org/10.4103/1673-5374.247465
Zhu JR et al (2018) Berberine attenuates ischemia-reperfusion injury through inhibiting HMGB1 release and NF-kappaB nuclear translocation. Acta Pharmacol Sin 39(11):1706–1715. https://doi.org/10.1038/s41401-018-0160-1
Zhao L et al (2021) Berberine attenuates cerebral ischemia-reperfusion injury induced neuronal apoptosis by down-regulating the CNPY2 signaling pathway. Front Pharmacol 12:609693. https://doi.org/10.3389/fphar.2021.609693
Pereira JF et al (2023) Palmatine, a natural alkaloid, attenuates memory deficits and neuroinflammation in mice submitted to permanent focal cerebral ischemia. J Neuroimmunol 381:578131. https://doi.org/10.1016/j.jneuroim.2023.578131
Acknowledgements
We are grateful for the professor for Yanan Sun for her technical support.
Funding
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (81974550), Scientific and Technological Innovation Project of China Academy of Chinese Medical Science (CI2021A04612, CI2021B017-03) and Fundamental Research Funds for the Central Public Welfare Research Institutes (ZZ13-YQ-046, ZXKT21007 and ZXKT23019).
Author information
Authors and Affiliations
Contributions
Jingjing Zhang: data curation, analysis, conceptualization, funding acquisition, investigation, writing—original draft; Liangliang Tian, Guangzhao Cao: data curation, investigation, visualization, software, validation; Xiaotong Zhu, Lihan Wang, Jingyi Hou, Yi Zhang, He Xu, Lixia Wang, Shicong Wang, Chen Zhao: data curation, investigation; Hongjun Yang: conceptualization, supervision, writing—review and editing. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were approved by Ethics Committee of Animal Care and Use of the Institute of Basic Theories for Chinese Medicine, Chinese Academy of Chinese Medical Sciences (No: IBTCMCACMS21-2203–05).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, L., Cao, G., Zhu, X. et al. Transcriptomics and Metabolomics Unveil the Neuroprotection Mechanism of AnGong NiuHuang (AGNH) Pill Against Ischaemic Stroke Injury. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04016-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04016-8