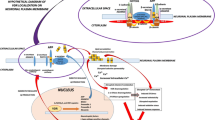
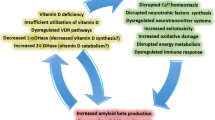
Abstract
Vitamin D3 (VD) is a secosteroid hormone and shows a pleiotropic effect in brain-related disorders where it regulates redox imbalance, inflammation, apoptosis, energy production, and growth factor synthesis. Vitamin D3’s active metabolic form, 1,25-dihydroxy Vitamin D3 (1,25(OH)2D3 or calcitriol), is a known regulator of several genes involved in neuroplasticity, neuroprotection, neurotropism, and neuroinflammation. Multiple studies suggest that VD deficiency can be proposed as a risk factor for the development of several age-related neurological disorders. The evidence for low serum levels of 25-hydroxy Vitamin D3 (25(OH)D3 or calcidiol), the major circulating form of VD, is associated with an increased risk of Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), dementia, and cognitive impairment. Despite decades of evidence on low VD association with neurological disorders, the precise molecular mechanism behind its beneficial effect remains controversial. Here, we will be delving into the neurobiological importance of VD and discuss its benefits in different neuropsychiatric disorders. The focus will be on AD, PD, and HD as they share some common clinical, pathological, and epidemiological features. The central focus will be on the different attributes of VD in the aspect of its anti-oxidative, anti-inflammatory, anti-apoptotic, anti-cholinesterase activity, and psychotropic effect in different neurodegenerative diseases.


Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- VD:
-
Vitamin D3
- mVDR:
-
Membrane Vitamin D receptor
- nVDR:
-
Nuclear Vitamin D receptor
- VDRE:
-
Vitamin D response element
- 1-alpha:
-
V25-dihydroxyvitamin D3 (1a,25-(OH)2D3) or calcitriol
- 25:
-
hydroxyvitamin D3 or 25(OH)D3 or calcidio
- GPCR:
-
G-protein-coupled receptors
- MAPK:
-
Mitogen-activated protein kinases
- ERK:
-
Extracellular signal-regulated kinase
- AKT:
-
Protein kinase B
- cAMP:
-
Cyclic adenosine monophosphate
- PKA:
-
Protein kinase A
- PLC:
-
Phospholipase C
- PIP2:
-
Phosphatidylinositol 4,5-bisphosphate
- DAG:
-
Diacylglycerol
- IP3:
-
1,4,5-Trisphosphate
- Ca+2 :
-
Calcium
- RXR:
-
Retinoid X receptor
- CREB:
-
CAMP response element-binding protein
- IL-10:
-
Interleukin 10
- IL-4:
-
Interleukin 4
- TGF:
-
β-Transforming growth factor-β
- BDNF:
-
Brain-derived neurotrophic factor
- NGF:
-
Nerve growth factor
- GDNF:
-
Glial cell line-derived neurotrophic factor
- NT-3:
-
Neurotrophin-3
- NT-4:
-
Neurotrophin-4
References
Nandi A, Counts N, Chen S et al (2022) Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: a value of statistical life approach. EClinicalMedicine 51:101580
Reynolds CF 3rd, Jeste DV, Sachdev PS, Blazer DG (2022) Mental health care for older adults: recent advances and new directions in clinical practice and research. World Psychiatry 21:336–363
He W, Deng Y, Luo X (2022) Bibliometric analysis of the global research status and trends of the association between vitamin D and infections from 2001 to 2021. Front Public Health 10:934106
Silberberg D, Anand NP, Michels K, Kalaria RN (2015) Brain and other nervous system disorders across the lifespan — global challenges and opportunities. Nature 527:S151–S154
Ding C, Wu Y, Chen X, Chen Y, Wu Z, Lin Z et al (2022) Global, regional, and national burden and attributable risk factors of neurological disorders: the global burden of disease study 1990–2019. Front Public Health 10:952161
Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N et al (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:459–480
GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7: e105–25.
Charlson F, van Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S (2019) New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. The Lancet 394:240–248
Béjot Y, Yaffe K (2019) Ageing population: a neurological challenge. Neuroepidemiology 52:76–77
Agnello L, Ciaccio M (2022) Neurodegenerative diseases: from molecular basis to therapy. Int J Mol Sci 23:12854
Baranov SV, Jauhari A, Carlisle DL, Friedlander RM (2021) Two hit mitochondrial-driven model of synapse loss in neurodegeneration. Neurobiol Diset 158:105451
Barry J, Bui MTN, Levine MS, Cepeda C (2022) Synaptic pathology in Huntington’s disease: beyond the corticostriatal pathway. Neurobiol Dis 162:105574
Behl T, Makkar R, Sehgal A, Singh S, Sharma N, Zengin G et al (2021) Current trends in neurodegeneration: cross talks between oxidative stress, cell death, and inflammation. Int J Mol Sci 22:7432
Berdenis van Berlekom A, Muflihah CH, Snijders GJLJ, MacGillavry HD, Middeldorp J, Hol EM et al (2020) Synapse pathology in schizophrenia: a meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull 46:374–86
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:32
Dugger BN, Dickson DW (2017) Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol 9:a028035
Shu Y, Su Q, Liao S, Lu T, Li R, Sun X et al (2017) Low serum vitamin D levels and anti-N-methyl-D-aspartate receptor encephalitis: a case-control study. Neurochem Int 102:89–94
Shen W, Zhai S, Surmeier DJ (2022) Striatal synaptic adaptations in Parkinson’s disease. Neurobiol Dis 167:105686
Stork C, Renshaw PF (2005) Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry 10:900–919
Wang G, Yin P, Zhang W, Giulietta Fernandez C, Xu X Editorial (2022) The relationship of neuroinflammation with aging and neurodegenerative diseases. Front Aging Neurosci 14:1102613
Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I (2023) Hallmarks of neurodegenerative diseases. Cell 186:693–714
Wingo TS, Liu Y, Gerasimov ES, Vattathil SM, Wynne ME, Liu J et al (2022) Shared mechanisms across the major psychiatric and neurodegenerative diseases. Nat Commun 13:4314
Zhao B, Schwartz JP (1998) Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. J Neurosci Res 52:7–16
Yang S, Park JH, Lu H-C (2023) Axonal energy metabolism, and the effects in aging and neurodegenerative diseases. Mol Neurodegener 18:49
Divo MJ, Martinez CH, Mannino DM (2014) Ageing and the epidemiology of multimorbidity. Eur Respir J 44:1055–1068
Weickenmeier J (2023) Exploring the multiphysics of the brain during development, aging, and in neurological diseases. Brain Multiphysics 4:100068
Dumurgier J, Tzourio C (2020) Epidemiology of neurological diseases in older adults. Rev Neurol (Paris) 176:642–648
Rehman MU, Wali AF, Ahmad A, Shakeel S, Rasool S, Ali R et al (2019) Neuroprotective strategies for neurological disorders by natural products: an update. Curr Neuropharmacol 17:247–267
Rehman MU, Sehar N, Dar NJ, Khan A, Arafah A, Rashid S et al (2023) Mitochondrial dysfunctions, oxidative stress and neuroinflammation as therapeutic targets for neurodegenerative diseases: an update on current advances and impediments. Neurosci Biobehav Rev 144:104961
Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F (2003) Vitamin D3 and brain development. Neuroscience 118:641–653
Cesari M, Incalzi RA, Zamboni V, Pahor M (2011) The vitamin D hormone: a multitude of actions potentially influencing the physical function decline in older persons. Geriatr Gerontol Int 11:133–142
Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J et al (2014) Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol 10:79–87
Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB (2022) The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol 18:96–110
Latimer CS, Brewer LD, Searcy JL, Chen K-C, Popović J, Kraner SD et al (2014) Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci U S A 111:E4359-4366
Mohamed AR, Soliman GY, Ismail CA, Mannaa HF (2015) Neuroprotective role of vitamin D3 in colchicine-induced Alzheimer’s disease in rats. Alex J Med 51:127–136
Berridge MJ (2017) Vitamin D deficiency accelerates ageing and age-related diseases: a novel hypothesis. J Physiol 595:6825–6836
Lima LAR, Lopes MJP, Costa RO, Lima FAV, Neves KRT, Calou IBF et al (2018) Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J Neuroinflammation 15:249
Koshkina A, Dudnichenko T, Baranenko D, Fedotova J, Drago F (2019) Effects of vitamin D3 in long-term ovariectomized rats subjected to chronic unpredictable mild stress: BDNF, NT-3, and NT-4 implications. Nutrients 11:1726
Mayne PE, Burne THJ (2019) Vitamin D in synaptic plasticity, cognitive function, and neuropsychiatric illness. Trends Neurosci 42:293–306
Alamro AA, Alsulami EA, Almutlaq M, Alghamedi A, Alokail M, Haq SH (2020) Therapeutic potential of vitamin D and curcumin in an in vitro model of Alzheimer disease. J Cent Nerv Syst Dis 12:1179573520924311
de Oliveira LRC, Mimura LAN, Fraga-Silva TF de C, Ishikawa LLW, Fernandes AAH, Zorzella-Pezavento SFG et al (2020) Calcitriol prevents neuroinflammation and reduces blood-brain barrier disruption and local macrophage/microglia activation. Front Pharmacol 11:161
Eyles DW (2021) Vitamin D: brain and behavior. JBMR Plus 5:e10419
Neriman A, Hakan Y, Ozge U (2021) The psychotropic effect of vitamin D supplementation on schizophrenia symptoms. BMC Psychiatry 21:309
Smolders J, Hiller A, Camu W (2021) Editorial: Vitamin D in neurological diseases from pathophysiology to therapy. Front Neurol 12:614900
Araújo de Lima L, Oliveira Cunha PL, Felicio Calou IB, Tavares Neves KR, Facundo HT, de Barros S, Viana G (2022) Effects of vitamin D (VD3) supplementation on the brain mitochondrial function of male rats, in the 6-OHDA-induced model of Parkinson’s disease. Neurochem Int 154:105280
Manjari SKV, Maity S, Poornima R, Yau S-Y, Vaishali K, Stellwagen D et al (2022) Restorative action of vitamin D3 on motor dysfunction through enhancement of neurotrophins and antioxidant expression in the striatum. Neurosci 492:67-81
Manjari S, Abraham SM, Poornima R, Chaturvedi RK, Maity S, Komal P (2023) Unprecedented effect of vitamin D3 on T-cell receptor beta subunit and alpha7 nicotinic acetylcholine receptor expression in a 3-nitropropionic acid induced mouse model of Huntington’s disease. IBRO Neurosci Rep 15:116–125
Pignolo A, Mastrilli S, Davì C, Arnao V, Aridon P, dos Santos Mendes FA et al (2022) Vitamin D and Parkinson’s disease. Nutrients 14:1220
Bivona G, Agnello L, Bellia C, Iacolino G, Scazzone C, Lo Sasso B et al (2019) Non-skeletal activities of vitamin D: from physiology to brain pathology. Medicina (Mex) 55:341
Anjum I, Jaffery SS, Fayyaz M, Samoo Z, Anjum S (2023) The role of vitamin D in brain health: a mini literature review. Cureus 10:e2960
Hashemi R, Morshedi M, Jafarabadi MA, Altafi D, Hosseini-Asl SS, Rafie-Arefhosseini S (2018) Anti-inflammatory effects of dietary vitamin D3 in patients with multiple sclerosis. Neurol Genet 4:e278
Berridge MJ (2016) Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos Trans R Soc B Biol Sci 371:20150434
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF et al (2008) Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29:726–776
Xu Y, Liang L (2021) Vitamin D3/vitamin D receptor signaling mitigates symptoms of post-stroke depression in mice by upregulating hippocampal BDNF expression. Neurosci Res 170:306–313
Ricca C, Aillon A, Bergandi L, Alotto D, Castagnoli C, Silvagno F (2018) Vitamin D receptor is necessary for mitochondrial function and cell health. Int J Mol Sci 19:1672
Sakai S, Suzuki M, Tashiro Y, Tanaka K, Takeda S, Aizawa K et al (2015) Vitamin D receptor signaling enhances locomotive ability in mice. J Bone Miner Res 30:128–136
Zmijewski MA, Carlberg C (2020) Vitamin D receptor(s): in the nucleus but also at membranes? Exp Dermatol 29:876–884
Zmijewski MA (2019) Vitamin D and human health. Int J Mol Sci 20:145
Yuan J, Guo X, Liu Z, Zhao X, Feng Y, Song S et al (2018) Vitamin D receptor activation influences the ERK pathway and protects against neurological deficits and neuronal death. Int J Mol Med 41:364–372
Cui X, Eyles DW (2022) Vitamin D and the central nervous system: causative and preventative mechanisms in brain disorders. Nutrients 14:4353
Przedborski S, Vila M, Jackson-Lewis V (2003) Series introduction: neurodegeneration: what is it and where are we? J Clin Invest 111:3–10
Fantini C, Corinaldesi C, Lenzi A, Migliaccio S, Crescioli C (2023) Vitamin D as a shield against aging. Int J Mol Sci 24:4546
Terock J, Bonk S, Frenzel S, Wittfeld K, Garvert L, Hosten N et al (2022) Vitamin D deficit is associated with accelerated brain aging in the general population. Psychiatry Res Neuroimaging 327:111558
Fullard ME, Duda JE (2020) A review of the relationship between vitamin D and Parkinson disease symptoms. Front Neurol 11:454
Soliman RH, Oraby MI, Hussein M, Abd El-Shafy S, Mostafa S (2019) Could vitamin D deficiency have an impact on motor and cognitive function in Parkinson’s disease? Egypt J Neurol Psychiatry Neurosurg 55:34
Chel VG, Ooms ME, van der Bent J, Veldkamp F, Roos RA, Achterberg WP et al (2013) High prevalence of vitamin D deficiency and insufficiency in patients with manifest Huntington disease. Dermatoendocrinol 5:348–351
Chai B, Gao F, Wu R, Dong T, Gu C, Lin Q et al (2019) Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: an updated meta-analysis. BMC Neurol 19:284
Holick MF (2018) The global D-Lemma: the vitamin D deficiency pandemic even in sun-drenched countries. J Clin Sci Res 7:101
Terock J, Bonk S, Frenzel S et al (2022) Vitamin D deficit is associated with accelerated brain aging in the general population. Psychiatry Res Neuroimaging 327:111558
Chen L, Yang R, Qiao W, Zhang W, Chen J, Mao L et al (2019) 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 18:e12951
Dursun E, Gezen-Ak D (2017) Vitamin D receptor is present on the neuronal plasma membrane and is co-localized with amyloid precursor protein, ADAM10 or Nicastrin. PLoS ONE 12:e0188605
Lasoń W, Jantas D, Leśkiewicz M, Regulska M, Basta-Kaim A (2023) The vitamin D receptor as a potential target for the treatment of age-related neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases: a narrative review. Cells 12:660
Bankole O, Laoye B, Sirajo MU, Ishola A, Oyeleke E, Balogun W et al (2015) Vitamin D 3 receptor activation rescued corticostriatal neural activity and improved motor function in –D 2 R tardive dyskinesia mice model. J Biomed Sci Eng. 8:520–30
Hii CS, Ferrante A (2016) The non-genomic actions of vitamin D. Nutrients 8:135
Wang W, Li Y, Meng X (2023) Vitamin D and neurodegenerative diseases. Heliyon 9:e12877
Yarlagadda K, Ma N, Doré S (2020) Vitamin D and stroke: effects on incidence, severity, and outcome and the potential benefits of supplementation. Front Neurol 11:384
Ghahremani M, Smith EE, Chen H-Y et al (2023) Vitamin D supplementation and incident dementia: effects of sex, APOE, and baseline cognitive status. Alzheimer’s & dementia: diagnosis, assessment & disease monitoring 15:e12404
McCollum EV, Simmonds N, Becker JE, Shipley PG (1922) Studies on experimental rickets: an experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem 53:293–312
Hess AF, Weinstock M (1924) Antirachitic properties imparted to inert fluids and to green vegetables by ultra-violet irradiation. J Biol Chem 62:301–313
Jones G (2022) 100 years of Vitamin D: historical aspects of vitamin D. Endocr Connect 11:e210594
Windans A, Bock F (1936) Über das Provitamin aus dem Sterin der Schweineschwarte 245:168–170
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G (2016) Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 96:365–408
Holick MF (1981) The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol 77:51–58
Jones G, Prosser DE, Kaufmann M (2014) Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 55:13–31
Norlin M, Wikvall K (2023) Enzymatic activation in vitamin D signaling - past, present and future. Arch Biochem Biophys 742:109639
Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW (2003) De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem 278:38084–38093
Seuter S, Ryynänen J, Carlberg C (2014) The ASAP2 gene is a primary target of 1,25-dihydroxyvitamin D3 in human monocytes and macrophages. J Steroid Biochem Mol Biol 144(Pt A):12–8
Cui X, Gooch H, Petty A, McGrath JJ, Eyles D (2017) Vitamin D and the brain: genomic and non-genomic actions. Mol Cell Endocrinol 453:131–143
Ekimoto T, Kudo T, Yamane T, Ikeguchi M (2021) Mechanism of vitamin D receptor ligand-binding domain regulation studied by gREST simulations. J Chem Inf Model 61:3625–3637
Cui X, Pertile R, Eyles DW (2018) The vitamin D receptor (VDR) binds to the nuclear matrix via its hinge domain: a potential mechanism for the reduction in VDR mediated transcription in mitotic cells. Mol Cell Endocrinol 472:18–25
Eyles DW (2020) Vitamin D: brain and behavior. JBMR Plus 5:e10419
de Angelis C, Galdiero M, Pivonello C, Garifalos F, Menafra D, Cariati F et al (2017) The role of vitamin D in male fertility: a focus on the testis. Rev Endocr Metab Disord 18:285–305
Habib AM, Nagi K, Thillaiappan NB, Sukumaran V, Akhtar S (2020) Vitamin D and its potential interplay with pain signaling pathways. Front Immunol 11:820
Bikle DD (2021) Ligand-independent actions of the vitamin D receptor: more questions than answers. JBMR Plus 1:211-216
Doroudi M, Schwartz Z, Boyan BD (2012) Phospholipase A2 activating protein is required for 1α,25-dihydroxyvitamin D3 dependent rapid activation of protein kinase C via Pdia3. J Steroid Biochem Mol Biol 132:48–56
Zaulkffali AS, Md Razip NN, Syed Alwi SS, Abd Jalil A, Abd Mutalib MS, Gopalsamy B et al (2019) Vitamins D and E stimulate the PI3K-AKT signalling pathway in insulin-resistant SK-N-SH neuronal cells. Nutrients 11:2525
Mizwicki MT, Menegaz D, Yaghmaei S, Henry HL, Norman AW (2010) A molecular description of ligand binding to the two overlapping binding pockets of the nuclear vitamin D receptor (VDR): structure-function implications. J Steroid Biochem Mol Biol 121:98–105
Warwick T, Schulz MH, Günther S, Gilsbach R, Neme A, Carlberg C et al (2021) A hierarchical regulatory network analysis of the vitamin D induced transcriptome reveals novel regulators and complete VDR dependency in monocytes. Sci Rep 11:6518
Alvarez N, Aguilar-Jimenez W, Rugeles MT (2019) The potential protective role of vitamin D supplementation on HIV-1 infection. Front Immunol 10:2291
Varghese JE, Balasubramanian B, Velayuthaprabhu S, Thirunavukkarasu V, Rengarajan RL, Murugesh E et al (2021) Therapeutic effects of vitamin D and cancer: an overview. Food Front Intern 2:417–25
Walker MD, Bilezikian JP (2017) Vitamin D and primary hyperparathyroidism: more insights into a complex relationship. Endocrine 55:3–5
Nakashima A, Yokoyama K, Yokoo T, Urashima M (2016) Role of vitamin D in diabetes mellitus and chronic kidney disease. World J Diabetes 7:89–100
Lemire JM, Archer DC (1991) 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest 87:1103–1107
Koduah P, Paul F, Dörr J-M (2017) Vitamin D in the prevention, prediction and treatment of neurodegenerative and neuroinflammatory diseases. EPMA J 8:313–325
Morello M, Landel V, Lacassagne E, Baranger K, Annweiler C, Féron F et al (2018) Vitamin D improves neurogenesis and cognition in a mouse model of Alzheimer’s disease. Mol Neurobiol 55:6463–6479
Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, Willett WC et al (2004) Vitamin D intake and incidence of multiple sclerosis. Neurology 62:60–65
Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A (2006) Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296:2832–2838
Feige J, Moser T, Bieler L, Schwenker K, Hauer L, Sellner J (2020) Vitamin D supplementation in multiple sclerosis: a critical analysis of potentials and threats. Nutrients 12:783
Gandhi F, Jhaveri S, Avanthika C, Singh A, Jain N, Gulraiz A et al (2023) Impact of vitamin D supplementation on multiple sclerosis. Cureus 13:e18487
Hiller AL, Murchison CF, Lobb BM, O’Connor S, O’Connor M, Quinn JF (2018) A randomized, controlled pilot study of the effects of vitamin D supplementation on balance in Parkinson’s disease: does age matter? PLoS ONE 13:e0203637
Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F (2019) Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry 90:1347–1352
Jia Q, Li S, Li X-J, Yin P (2022) Neuroinflammation in Huntington’s disease: from animal models to clinical therapeutics. Front Immunol 13:1088124
Annweiler C (2016) Vitamin D in dementia prevention. Ann N Y Acad Sci 1367:57–63
Annweiler C, Beauchet O (2011) Vitamin D-mentia: randomized clinical trials should be the next step. Neuroepidemiology 37:249–258
Annweiler C, Schott A-M, Berrut G, Chauviré V, Le Gall D, Inzitari M et al (2010) Vitamin D and ageing: neurological issues. Neuropsychobiology 62:139–150
Anwar MJ, Alenezi SK, Alhowail AH (2023) Molecular insights into the pathogenic impact of vitamin D deficiency in neurological disorders. Biomed Pharmacother Biomedecine Pharmacother 162:114718
Singh P, Mishra G, Molla M, Shumet Yimer Y, Sisay W, Andargie Y et al (2022) Dietary and nutraceutical-based therapeutic approaches to combat the pathogenesis of Huntington’s disease. J Funct Foods 92:105047
Yamini P, Ray RS, Chopra K (2018) Vitamin D3 attenuates cognitive deficits and neuroinflammatory responses in ICV-STZ induced sporadic Alzheimer’s disease. Inflammopharmacology 26:39–55
Mehrabadi S, Sadr SS (2020) Administration of Vitamin D3 and E supplements reduces neuronal loss and oxidative stress in a model of rats with Alzheimer’s disease. Neurol Res 42:862–868
Patel P, Shah J (2022) Vitamin D3 supplementation ameliorates cognitive impairment and alters neurodegenerative and inflammatory markers in scopolamine induced rat model. Metab Brain Dis 37:2653–2667
Magdy A, Farrag EAE, Hamed SM, Abdallah Z, El Nashar EM, Alghamdi MA et al (2022) Neuroprotective and therapeutic effects of calcitriol in rotenone-induced Parkinson’s disease rat model. Front Cell Neurosci 16:967813
Calvello R, Cianciulli A, Nicolardi G, De Nuccio F, Giannotti L, Salvatore R et al (2017) Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol Off J Soc NeuroImmune Pharmacol 12:327–339
Fort Molnár M, Török R, Sümegi E, Vécsei L, Klivényi P, Szalárdy L (2016) High-dose 1,25-dihydroxyvitamin D supplementation elongates the lifespan of Huntington’s disease transgenic mice. Acta Neurobiol Exp (Warsz) 76:176–81
Komal P, Manjari SKV, Nashmi R (2022) An opinion on the debatable function of brain resident immune protein, T-cell receptor beta subunit in the central nervous system. IBRO Neurosci Rep 13:235–242
Landel V, Annweiler C, Millet P, Morello M, Féron F, Vitamin D (2016) Cognition and Alzheimer’s disease: the therapeutic benefit is in the D-tails. J Alzheimers Dis JAD 53:419–444
Boucher BJ (2012) The problems of vitamin D insufficiency in older people. Aging Dis 3:313–329
Goodwill AM, Szoeke C (2017) A systematic review and meta-analysis of the effect of low vitamin D on cognition. J Am Geriatr Soc 65:2161–2168
Sultan S, Taimuri U, Basnan SA, Ai-Orabi WK, Awadallah A, Almowald F et al (2020) Low vitamin D and its association with cognitive impairment and dementia. J Aging Res 2020:1–10
Gaughran F, Stringer D, Wojewodka G, Landau S, Smith S, Gardner-Sood P et al (2021) Effect of vitamin D supplementation on outcomes in people with early psychosis: the DFEND randomized clinical trial. JAMA Netw Open 4:e2140858
Khadilkar A, Kajale N, Oza C, Oke R, Gondhalekar K, Patwardhan V et al (2022) Vitamin D status and determinants in Indian children and adolescents: a multicentre study. Sci Rep 12:16790
Holick MF, Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080S-1086S
Holick MF, Vitamin D (2009) Status: measurement, interpretation and clinical application. Ann Epidemiol 19:73–78
Dawson-Hughes B, Mithal A, Bonjour J-P, Boonen S, Burckhardt P, Fuleihan GE-H et al (2010) IOF position statement: vitamin D recommendations for older adults. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 21:1151–4
Azam S, Haque MdE, Balakrishnan R, Kim I-S, Choi D-K (2021) The ageing brain: molecular and cellular basis of neurodegeneration. Front Cell Dev Biol 9:683459
Banerjee A, Khemka VK, Ganguly A, Roy D, Ganguly U, Chakrabarti S (2015) Vitamin D and Alzheimer’s disease: neurocognition to therapeutics. Int J Alzheimers Dis 2015:1–11
Somoza-Moncada MM, Turrubiates-Hernández FJ, Muñoz-Valle JF, Gutiérrez-Brito JA, Díaz-Pérez SA, Aguayo-Arelis A et al (2023) Vitamin D in depression: a potential bioactive agent to reduce suicide and suicide attempt risk. Nutrients 15:1765
Almeras L, Eyles D, Benech P, Laffite D, Villard C, Patatian A et al (2007) Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics 7:769–780
Banerjee P, Chatterjee M (2003) Antiproliferative role of vitamin D and its analogs–a brief overview. Mol Cell Biochem 253:247–254
Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay-Sim A, Burne THJ (2013) Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res 241:120–131
Kasatkina L, Tarasenko A, Krupko O, Kuchmerovska T, Lisakovska O, Trikash I (2019) Vitamin D deficiency induces the excitation/inhibition brain imbalance and the proinflammatory shift. Int J Biochem Cell Biol 119:105665
Kim HK, Andreazza AC, Yeung PY, Isaacs-Trepanier C, Young LT (2014) Oxidation and nitration in dopaminergic areas of the prefrontal cortex from patients with bipolar disorder and schizophrenia. J Psychiatry Neurosci JPN 39:276–285
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat 29:21–30
Van Cromphaut SJ, Dewerchin M, Hoenderop JGJ, Stockmans I, Van Herck E, Kato S et al (2001) Duodenal calcium absorption in vitamin D receptor–knockout mice: functional and molecular aspects. Proc Natl Acad Sci 98:13324–13329
Burne THJ, McGrath JJ, Eyles DW, Mackay-Sim A (2005) Behavioural characterization of vitamin D receptor knockout mice. Behav Brain Res 157:299–308
Christakos S, Seth T, Hirsch J, Porta A, Moulas A, Dhawan P (2013) Vitamin D biology revealed through the study of knockout and transgenic mouse models. Annu Rev Nutr 33:71–85
Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y et al (1997) Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16:391–396
Gezen-Ak D, Dursun E, Yilmazer S (2011) The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS ONE 6:e17553
Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S (2010) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70:271–288
Duarte Azevedo M, Sander S, Tenenbaum L (2020) GDNF, A NEURON-DERIVED FACTOR UPREGULATED IN GLIAL CELLS DURING DISEASE. J Clin Med 9:456
Wion D, Macgrogan D, Neveu I, Jehan F, Houlgatte R, Brachet P (1991) 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res 28:110–114
Naveilhan P, Neveu I, Wion D, Brachet P (1996) 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. NeuroReport 7:2171–2175
Baydyuk M, Xu B (2014) BDNF signaling and survival of striatal neurons. Front Cell Neurosci 8:254
Abiri B, Vafa M (2020) Effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammatory biomarkers, and SIRT1 in obese women: a study protocol for a double-blind, randomized, placebo-controlled trial. Trials 21:225
Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J (2018) BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol 38:579–593
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138:155–175
Brown J, Bianco JI, McGrath JJ, Eyles DW (2003) 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett 343:139–143
Cornet A, Baudet C, Neveu I, Baron-Van Evercooren A, Brachet P, Naveilhan P (1998) 1,25-dihydroxyvitamin D3 regulates the expression of VDR and NGF gene in Schwann cells in vitro. J Neurosci Res 53:742–746
Nadimi H, Djazayery A, Javanbakht MH, Dehpour A, Ghaedi E, Derakhshanian H et al (2020) Effect of vitamin D supplementation on CREB-TrkB-BDNF pathway in the hippocampus of diabetic rats. Iran J Basic Med Sci 23:117–123
Alsulami E, Alokail M, Alghamedi A, Alamro A, Haq S (2020) Effect of vitamin D treatment on VDR expression in primary cerebral cortical cells in induced oxidative stress. J Cell Biotechnol 6:81–90
Pertile RAN, Cui X, Hammond L, Eyles DW (2018) Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J Off Publ Fed Am Soc Exp Biol 32:819–828
Bao Z, Wang X, Li Y, Feng F (2020) Vitamin D alleviates cognitive dysfunction by activating the VDR/ERK1/2 signaling pathway in an Alzheimer’s disease mouse model. NeuroImmunoModulation 27:178–185
Haussler MR, Haussler CA, Whitfield GK, Hsieh J-C, Thompson PD, Barthel TK et al (2010) The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol 121:88–97
Pérez-Navarro E, Canudas AM, Akerund P, Alberch J, Arenas E (2000) Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 prevent the death of striatal projection neurons in a rodent model of Huntington’s disease. J Neurochem 75:2190–2199
Lee PW, Selhorst A, Lampe SG, Liu Y, Yang Y, Lovett-Racke AE (2020) Neuron-specific vitamin D signaling attenuates microglia activation and CNS autoimmunity. Front Neurol 11:19
Mazzetti S, Barichella M, Giampietro F, Giana A, Calogero AM, Amadeo A et al (2022) Astrocytes expressing vitamin D-activating enzyme identify Parkinson’s disease. CNS Neurosci Ther 28:703–713
Jiao K-P, Li S-M, Lv W-Y, Jv M-L, He H-Y (2017) Vitamin D3 repressed astrocyte activation following lipopolysaccharide stimulation in vitro and in neonatal rats. NeuroReport 28:492–497
Alessio N, Belardo C, Trotta MC, Paino S, Boccella S, Gargano F et al (2021) Vitamin D deficiency induces chronic pain and microglial phenotypic changes in mice. Int J Mol Sci 22:3604
Khairy EY, Attia MM (2021) Protective effects of vitamin D on neurophysiologic alterations in brain aging: role of brain-derived neurotrophic factor (BDNF). Nutr Neurosci 24:650–659
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Levy MJF, Boulle F, Steinbusch HW, van den Hove DLA, Kenis G, Lanfumey L (2018) Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 235:2195–2220
Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13:363
Gao L, Zhang Y, Sterling K, Song W (2022) Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl Neurodegener 11:4
Castrén E, Antila H (2017) Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry 22:1085–1095
Gibon J, Barker PA (2017) Neurotrophins and proneurotrophins: focus on synaptic activity and plasticity in the brain. Neuroscientist 23:587–604
Blesch A (2006) Neurotrophic factors in neurodegeneration. Brain Pathol 16:295–303
Ma B, Culver BP, Baj G, Tongiorgi E, Chao MV, Tanese N (2010) Localization of BDNF mRNA with the Huntington’s disease protein in rat brain. Mol Neurodegener 5:22
Xie Y, Hayden MR, Xu B (2010) BDNF Overexpression in the Forebrain Rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci 30:14708–14718
AlJohri R, AlOkail M, Haq SH (2019) Neuroprotective role of vitamin D in primary neuronal cortical culture. eNeurologicalSci 14:43–8
Bayo-Olugbami A, Nafiu AB, Amin A, Ogundele OM, Lee CC, Owoyele BV (2020) Vitamin D attenuated 6-OHDA-induced behavioural deficits, dopamine dysmetabolism, oxidative stress, and neuro-inflammation in mice. Nutr Neurosci 25:823–834
Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN et al (2010) Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest 120:1774–1785
Atif F, Yousuf S, Sayeed I, Ishrat T, Hua F, Stein D (2012) Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: the role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacology 67:78–87
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58:621–631
Bakhtiari-Dovvombaygi H, Izadi S, Zare M, Asgari Hassanlouei E, Dinpanah H, Ahmadi-Soleimani SM et al (2021) Vitamin D3 administration prevents memory deficit and alteration of biochemical parameters induced by unpredictable chronic mild stress in rats. Sci Rep 11:16271
Liang Q, Cai C, Duan D, Hu X, Hua W, Jiang P et al (2018) Postnatal vitamin D intake modulates hippocampal learning and memory in adult mice. Front Neurosci 12:141
Gezen-Ak D, Yılmazer S, Dursun E (2014) Why vitamin D in Alzheimer’s disease? The hypothesis J Alzheimers Dis 40:257–269
Marlin MC, Li G (2015) Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol 314:239–257
Yang H, Wang L, Zang C, Wang Y, Shang J, Zhang Z et al (2020) Src inhibition attenuates neuroinflammation and protects dopaminergic neurons in Parkinson’s disease models. Front Neurosci 14:45
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci 361:1545–1564
Minnone G, De Benedetti F, Bracci-Laudiero L (2017) NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci 18:1028
Naveilhan P, Neveu I, Baudet C, Funakoshi H, Wion D, Brachet P et al (1996) 1,25-Dihydroxyvitamin D3 regulates the expression of the low-affinity neurotrophin receptor. Mol Brain Res 41:259–268
Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang G-X (2015) 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol 98:240–245
Proenca CC, Song M, Lee FS (2016) Differential effects of BDNF and neurotrophin 4 (NT4) on endocytic sorting of TrkB receptors. J Neurochem 138:397–406
Khan N, Smith M (2015) Neurotrophins and neuropathic pain: role in pathobiology. Molecules 20:10657–10688
Yan Z, Shi X, Wang H, Si C, Liu Q, Du Y (2021) Neurotrophin-3 promotes the neuronal differentiation of BMSCs and improves cognitive function in a rat model of Alzheimer’s disease. Front Cell Neurosci 15:629356
Lara-Rodarte R, Cortés D, Soriano K, Carmona F, Rocha L, Estudillo E et al (2021) Mouse embryonic stem cells expressing gdnf show enhanced dopaminergic differentiation and promote behavioral recovery after grafting in Parkinsonian rats. Front Cell Dev Biol 9:661656
Gattei V, Celetti A, Cerrato A, Degan M, De Iuliis A, Rossi FM et al (1997) Expression of the RET receptor tyrosine kinase and GDNFR-α in normal and leukemic human hematopoietic cells and stromal cells of the bone marrow microenvironment. Blood 89:2925–2937
Stanga S, Brambilla L, Tasiaux B, Dang AH, Ivanoiu A, Octave J-N et al (2018) A role for GDNF and soluble APP as biomarkers of amyotrophic lateral sclerosis pathophysiology. Front Neurol 9:384
Bahlakeh G, Rahbarghazi R, Mohammadnejad D, Elahi A, Karimipour M (2021) Current knowledge and challenges associated with targeted delivery of neurotrophic factors into the central nervous system; focus on available approaches. Cell Biosci 11:181
Goldberg P (1974) Multiple sclerosis: vitamin D and calcium as environmental determinants of prevalence. Int J Environ Stud 6:121–129
Mark KA, Dumas KJ, Bhaumik D, Schilling B, Davis S, Oron TR et al (2016) Vitamin D Promotes Protein Homeostasis And Longevity Via The Stress Response Pathway Genes skn-1, ire-1, and xbp-1. Cell Rep 17:1227–1237
Durk MR, Han K, Chow ECY, Ahrens R, Henderson JT, Fraser PE et al (2014) 1α,25-Dihydroxyvitamin D3 reduces cerebral amyloid-β accumulation and improves cognition in mouse models of Alzheimer’s disease. J Neurosci Off J Soc Neurosci 34:7091–7101
Gezen-Ak D, Alaylıoğlu M, Yurttaş Z, Çamoğlu T, Şengül B, İşler C et al (2023) Vitamin D receptor regulates transcription of mitochondrial DNA and directly interacts with mitochondrial DNA and TFAM. J Nutr Biochem 116:109322
Zhou Z, Zhou R, Zhang Z, Li K (2019) The association between vitamin D status, vitamin D supplementation, sunlight exposure, and Parkinson’s disease: a systematic review and meta-analysis. Med Sci Monit Int Med J Exp Clin Res 25:666–674
Berg D, Youdim MBH, Riederer P (2004) Redox imbalance. Cell Tissue Res 318:201–213
Rey F, Berardo C, Maghraby E, Mauri A, Messa L, Esposito L et al (2023) Redox imbalance in neurological disorders in adults and children. Antioxidants 12:965
Agnihotri A, Aruoma OI (2020) Alzheimer’s disease and Parkinson’s disease: a nutritional toxicology perspective of the impact of oxidative stress, mitochondrial dysfunction, nutrigenomics and environmental chemicals. J Am Coll Nutr 39:16–27
Rikani AA, Choudhry Z, Choudhry AM, Rizvi N, Ikram H, Mobassarah NJ et al (2014) The mechanism of degeneration of striatal neuronal subtypes in Huntington disease. Ann Neurosci 21:112–114
Rotermund C, Machetanz G, Fitzgerald JC (2018) The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol 9:400
Collin F (2019) Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int J Mol Sci 20:2407
Kim TY, Leem E, Lee JM, Kim SR (2020) Control of reactive oxygen species for the prevention of Parkinson’s disease: the possible application of flavonoids. Antioxidants 9:583
Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M (2000) Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20:2175–2183
Saad El-Din S, Rashed L, Medhat E, Emad Aboulhoda B, Desoky Badawy A, Mohammed ShamsEldeen A et al (2020) Active form of vitamin D analogue mitigates neurodegenerative changes in Alzheimer’s disease in rats by targeting Keap1/Nrf2 and MAPK-38p/ERK signaling pathways. Steroids 156:108586
Brandes MS, Gray NE (2020) NRF2 as a therapeutic target in neurodegenerative diseases. ASN Neuro 12:1759091419899782
da Costa RO, Gadelha-Filho CVJ, de Aquino PEA, Lima LAR, de Lucena JD, Ribeiro WLC et al (2023) Vitamin D (VD3) Intensifies the effects of exercise and prevents alterations of behavior, brain oxidative stress, and neuroinflammation, in Hemiparkinsonian rats. Neurochem Res 48:142–160
Zhang Y, Ji W, Zhang S, Gao N, Xu T, Wang X et al (2022) Vitamin D inhibits the early aggregation of α‐synuclein and modulates exocytosis revealed by electrochemical measurements. Angew Chem Int Ed Engl 61:e202111853
Shinpo K, Kikuchi S, Sasaki H, Moriwaka F, Tashiro K (2000) Effect of 1,25-dihydroxyvitamin D3 on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L-buthionine sulfoximine and 1-methyl-4-phenylpyridine. J Neurosci Res 62:374–382
Matta Reddy A, Iqbal M, Chopra H, Urmi S, Junapudi S, Bibi S et al (2022) Pivotal role of vitamin D in mitochondrial health, cardiac function, and human reproduction. Excli j 21:967–990
Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH (2010) Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta BBA - Mol Basis Dis 1802:228–234
Garcion E, Nataf S, Berod A, Darcy F, Brachet P (1997) 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Mol Brain Res 45:255–267
Garcion E, Sindji L, Leblondel G, Brachet P, Darcy F (2002) 1,25-Dihydroxyvitamin D3 regulates the synthesis of γ-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J Neurochem 73:859–866
Wang W, Li Y, Meng X (2023) Vitamin D and neurodegenerative diseases. Heliyon 9:e12877
Jain SK, Parsanathan R, Achari AE, Kanikarla-Marie P, Bocchini JA (2018) Glutathione stimulates vitamin D regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25-hydroxy-vitamin D levels in blood: a novel approach to treat 25-hydroxyvitamin D deficiency. Antioxid Redox Signal 29:1792–1807
Brown AJ, Slatopolsky E (2008) Vitamin D analogs: therapeutic applications and mechanisms for selectivity. Mol Aspects Med 29:433–452
Taniura H, Ito M, Sanada N, Kuramoto N, Ohno Y, Nakamichi N et al (2006) Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosci Res 83:1179–1189
Cui C, Xu P, Li G, Qiao Y, Han W, Geng C et al (2019) Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol 26:101295
Jamilian H, Amirani E, Milajerdi A, Kolahdooz F, Mirzaei H, Zaroudi M et al (2019) The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: a systematic review and meta-analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry 94:109651
Lin C-I, Chang Y-C, Kao N-J, Lee W-J, Cross T-W, Lin S-H (2020) 1,25(OH)2D3 Alleviates Aβ (25–35)-induced tau hyperphosphorylation, excessive reactive oxygen species, and apoptosis through interplay with glial cell line-derived neurotrophic factor signaling in SH-SY5Y cells. Int J Mol Sci 21:4215
Hu J, Wu J, Wan F, Kou L, Yin S, Sun Y et al (2021) Calcitriol Alleviates MPP+- and MPTP-induced parthanatos through the VDR/PARP1 pathway in the model of Parkinson’s disease. Front Aging Neurosci 13:657095
Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M et al (2009) PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem 284:21379–21385
Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT et al (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 26:3169–3179
Hasegawa K, Yoshikawa K (2008) Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J Neurosci Off J Soc Neurosci 28:8772–8784
Chen J, Zhou Y, Mueller-Steiner S, Chen L-F, Kwon H, Yi S et al (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem 280:40364–40374
Mello-Filho AC, Meneghini R (1991) Iron is the intracellular metal involved in the production of DNA damage by oxygen radicals. Mutat Res Mol Mech Mutagen 251:109–113
Zhen C, Feng X, Li Z, Wang Y, Li B, Li L et al (2015) Suppression of murine experimental autoimmune encephalomyelitis development by 1,25-dihydroxyvitamin D3 with autophagy modulation. J Neuroimmunol 280:1–7
Şahin S, Gürgen SG, Yazar U, İnce İ, Kamaşak T, Acar Arslan E et al (2019) Vitamin D protects against hippocampal apoptosis related with seizures induced by kainic acid and pentylenetetrazol in rats. Epilepsy Res 149:107–116
Uberti F, Lattuada D, Morsanuto V, Nava U, Bolis G, Vacca G et al (2014) Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J Clin Endocrinol Metab 99:1367–1374
Kim J-S, Ryu S-Y, Yun I, Kim W-J, Lee K-S, Park J-W et al (2006) 1α,25-Dihydroxyvitamin D3 protects dopaminergic neurons in rodent models of Parkinson’s disease through inhibition of microglial activation. J Clin Neurol Seoul Korea 2:252–257
Lefebvre d’Hellencourt C, Montero-Menei CN, Bernard R, Couez D (2003) Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res 71:575–582
Doncheva N, Mihaylova A, Zlatanova H, Ivanovska M, Delev D, Murdjeva M et al (2022) Vitamin D3 exerts immunomodulatory and memory improving properties in rats with lipopolysaccharide-induced inflammation. Folia Med (Plovdiv) 64:770–781
Evans MA, Kim HA, Ling YH, Uong S, Vinh A, De Silva TM et al (2018) Vitamin D3 supplementation reduces subsequent brain injury and inflammation associated with ischemic stroke. NeuroMolecular Med 20:147–159
Verma R, Kim JY (2016) 1,25-Dihydroxyvitamin D3 facilitates M2 polarization and upregulates TLR10 expression on human microglial cells. NeuroImmunoModulation 23:75–80
Nissou M-F, Guttin A, Zenga C, Berger F, Issartel J-P, Wion D (2014) Additional clues for a protective role of vitamin D in neurodegenerative diseases: 1,25-dihydroxyvitamin D3 triggers an anti-inflammatory response in brain pericytes. J Alzheimers Dis JAD 42:789–799
Galoppin M, Kari S, Soldati S, Pal A, Rival M, Engelhardt B et al (2022) Full spectrum of vitamin D immunomodulation in multiple sclerosis: mechanisms and therapeutic implications. Brain Commun 4:171
Rastegar-Moghaddam SH, Hosseini M, Alipour F, Rajabian A, Ebrahimzadeh BA (2022) The effects of vitamin D on learning and memory of hypothyroid juvenile rats and brain tissue acetylcholinesterase activity and oxidative stress indicators. Naunyn Schmiedebergs Arch Pharmacol 395:337–351
Al-Zahrani YA, Sattar MAAA, Al-Harthi SE, Alkatheeri AA, Al-Zahrani YM (2021) Vitamin D3 attenuates type 3 diabetic-associated cognitive deficits in rats through regulating neurotrophins and enhancing cholinergic transmission pathway. J Pharmacol Pharmacother 12:47–53
Kumar PT, Antony S, Nandhu MS, Sadanandan J, Naijil G, Paulose CS (2011) Vitamin D3 restores altered cholinergic and insulin receptor expression in the cerebral cortex and muscarinic M3 receptor expression in pancreatic islets of streptozotocin induced diabetic rats. J Nutr Biochem 22:418–425
Rodrigues MV, Gutierres JM, Carvalho F, Lopes TF, Antunes V, da Costa P et al (2019) Protection of cholinergic and antioxidant system contributes to the effect of Vitamin D3 ameliorating memory dysfunction in sporadic dementia of Alzheimer’s type. Redox Rep Commun Free Radic Res 24:34–40
Huang Q, Liao C, Ge F, Ao J, Liu T (2022) Acetylcholine bidirectionally regulates learning and memory. J Neurorestoratology 10:100002
Tata AM, Velluto L, D’Angelo C, Reale M (2014) Cholinergic system dysfunction and neurodegenerative diseases: cause or effect? CNS Neurol Disord Drug Targets 13:1294–1303
Perez-Lloret S, Barrantes FJ (2016) Deficits in cholinergic neurotransmission and their clinical correlates in Parkinson’s disease. Npj Park Dis 2:1–12
Komal P, Estakhr J, Kamran M, Renda A, Nashmi R (2015) cAMP-dependent protein kinase inhibits α7 nicotinic receptor activity in layer 1 cortical interneurons through activation of D1/D5 dopamine receptors. J Physiol 593:3513–3532
Komal P, Gudavicius G, Nelson CJ, Nashmi R (2014) T-cell receptor activation decreases excitability of cortical interneurons by inhibiting α7 nicotinic receptors. J Neurosci Off J Soc Neurosci 34:22–35
Winek K, Soreq H, Meisel A (2021) Regulators of cholinergic signaling in disorders of the central nervous system. J Neurochem 158:1425–1438
Kisby B, Jarrell JT, Agar ME, Cohen DS, Rosin ER, Cahill CM et al (2019) Alzheimer’s disease and its potential alternative therapeutics. J Alzheimers Dis Parkinsonism 9:477
Caton M, Ochoa ELM, Barrantes FJ (2020) The role of nicotinic cholinergic neurotransmission in delusional thinking. Npj Schizophr 6:1–12
Chen Z-R, Huang J-B, Yang S-L, Hong F-F (2022) Role of cholinergic signaling in Alzheimer’s disease. Molecules 27:1816
Iarkov A, Mendoza C, Echeverria V (2021) Cholinergic receptor modulation as a target for preventing dementia in Parkinson’s disease. Front Neurosci 15:665820
Hampel H, Mesulam M-M, Cuello AC, Farlow MR, Giacobini E, Grossberg GT et al (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141:1917–1933
Assous M (2021) Striatal cholinergic transmission. Focus on nicotinic receptors’ influence in striatal circuits. Eur J Neurosci 53:2421–42
Conejero-Goldberg C, Davies P, Ulloa L (2008) Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci Biobehav Rev 32:693–706
Foucault - Fruchard L, Doméné A, Page G, Windsor M, Emond P, Rodrigues N et al (2017) Neuroprotective effect of the alpha 7 nicotinic receptor agonist PHA 543613 in an in-vivo excitotoxic adult rat model. Neuroscience 356:52–63
Papke RL, Horenstein NA (2021) Therapeutic targeting of α7 nicotinic acetylcholine receptors. Pharmacol Rev 73:1118–1149
Tregellas JR, Wylie KP (2019) Alpha7 Nicotinic receptors as therapeutic targets in schizophrenia. Nicotine Tob Res Off J Soc Res Nicotine Tob 21:349–356
Ma K-G, Qian Y-H (2019) Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer’s disease. Neuropeptides 73:96–106
Komal P, Evans G, Nashmi R (2011) A rapid agonist application system for fast activation of ligand-gated ion channels. J Neurosci Methods 198:246–254
Dau A, Komal P, Truong M, Morris G, Evans G, Nashmi R (2013) RIC-3 differentially modulates α4β2 and α7 nicotinic receptor assembly, expression, and nicotine-induced receptor upregulation. BMC Neurosci 14:47
Elseweidy MM, Mahrous M, Ali SI, Shaheen MA, Younis NN (2023) Vitamin D alleviates cognitive dysfunction and brain damage induced by copper sulfate intake in experimental rats: focus on its combination with donepezil. Naunyn Schmiedebergs Arch Pharmacol 396(9):1931–1942
Alrefaie Z, Alhayani A (2015) Vitamin D3 improves decline in cognitive function and cholinergic transmission in prefrontal cortex of streptozotocin-induced diabetic rats. Behav Brain Res 287:156–162
Karabulut M, Baykan O, Aksoz E (2021) Age-related differences in the effect of vitamin D on scopolamine-induced learning and memory impairment. Ann Med Res 28:799
Wu B, Tao X, Liu C, Li H, Jiang T, Chen Z et al (2021) Vitamin D3 reduces hippocampal NR2A and anxiety in nicotine withdrawal mice. Transl Neurosci 12:273–281
Marino A, Battaglini M, Moles N, Ciofani G (2022) Natural antioxidant compounds as potential pharmaceutical tools against neurodegenerative diseases. ACS Omega 7:25974–25990
Muller M, Leavitt BR (2014) Iron dysregulation in Huntington’s disease. J Neurochem 130:328–350
Wan W, Jin L, Wang Z, Wang L, Fei G, Ye F et al (2017) Iron deposition leads to neuronal α-synuclein pathology by inducing autophagy dysfunction. Front Neurol 8:1
Chen B, Wen X, Jiang H, Wang J, Song N, Xie J (2019) Interactions between iron and α-synuclein pathology in Parkinson’s disease. Free Radic Biol Med 141:253–260
Mi Y, Gao X, Xu H, Cui Y, Zhang Y, Gou X (2019) The emerging roles of ferroptosis in Huntington’s disease. Neuromolecular Med 21:110–119
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072
Hassannia B, Van Coillie S, Vanden BT (2021) Ferroptosis: biological rust of lipid membranes. Antioxid Redox Signal 35:487–509
David S, Jhelum P, Ryan F, Jeong SY, Kroner A (2022) Dysregulation of iron homeostasis in the central nervous system and the role of ferroptosis in neurodegenerative disorders. Antioxid Redox Signal 37:150–170
Yang WS, Stockwell BR (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26:165–176
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285
Jhelum P, Santos-Nogueira E, Teo W, Haumont A, Lenoël I, Stys PK et al (2020) Ferroptosis mediates cuprizone-induced loss of oligodendrocytes and demyelination. J Neurosci 40:9327–9341
Jhelum P, Zandee S, Ryan F, Zarruk JG, Michalke B, Venkataramani V et al (2023) Ferroptosis induces detrimental effects in chronic EAE and its implications for progressive MS. Acta Neuropathol Commun 11:121
Bao W-D, Pang P, Zhou X-T, Hu F, Xiong W, Chen K et al (2021) Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ 28:1548–1562
Conde JR, Streit WJ (2006) Microglia in the aging brain. J Neuropathol Exp Neurol 65:199–203
Jeong SY, David S (2006) Age-related changes in iron homeostasis and cell death in the cerebellum of ceruloplasmin-deficient mice. J Neurosci Off J Soc Neurosci 26:9810–9819
Farrall AJ, Wardlaw JM (2009) Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging 30:337–352
Rouault TA, Tong W-H (2005) Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol 6:345–351
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117:285–297
Rouault TA (2006) The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol 2:406–414
Hu Z, Zhang H, Yi B, Yang S, Liu J, Hu J et al (2020) VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis 11:1–11
Li L, Li W-J, Zheng X-R, Liu Q-L, Du Q, Lai Y-J et al (2022) Eriodictyol ameliorates cognitive dysfunction in APP/PS1 mice by inhibiting ferroptosis via vitamin D receptor-mediated Nrf2 activation. Mol Med Camb Mass 28:11
Rigby WF, Denome S, Fanger MW (1987) Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 79:1659–64
Claro da Silva T, Hiller C, Gai Z, Kullak-Ublick GA (2016) Vitamin D3 transactivates the zinc and manganese transporter SLC30A10 via the Vitamin D receptor. J Steroid Biochem Mol Biol 163:77–87
Molinari C, Morsanuto V, Ghirlanda S, Ruga S, Notte F, Gaetano L et al (2019) Role of combined lipoic acid and vitamin D3 on astrocytes as a way to prevent brain ageing by induced oxidative stress and iron accumulation. Oxid Med Cell Longev 2019:2843121
Wu T-Y, Zhao L-X, Zhang Y-H, Fan Y-G (2022) Activation of vitamin D receptor inhibits Tau phosphorylation is associated with reduction of iron accumulation in APP/PS1 transgenic mice. Neurochem Int 153:105260
Bennett L, Kersaitis C, Macaulay SL, Münch G, Niedermayer G, Nigro J et al (2013) Vitamin D2-enriched button mushroom (Agaricus bisporus) improves memory in both wild type and APPswe/PS1dE9 transgenic mice. PLoS ONE 8:e76362
Mehri N, Haddadi R, Ganji M, Shahidi S, Soleimani Asl S, Taheri Azandariani M et al (2020) Effects of vitamin D in an animal model of Alzheimer’s disease: behavioral assessment with biochemical investigation of Hippocampus and serum. Metab Brain Dis 35:263–274
Chinta SJ, Andersen JK (2008) Redox imbalance in Parkinson’s disease. Biochim Biophys Acta 1780:1362–1367
Jang W, Kim HJ, Li H, Jo KD, Lee MK, Song SH et al (2014) 1,25-Dyhydroxyvitamin D3 attenuates rotenone-induced neurotoxicity in SH-SY5Y cells through induction of autophagy. Biochem Biophys Res Commun 451:142–147
Jang W, Park H-H, Lee K-Y, Lee YJ, Kim H-T, Koh S-H (2015) 1,25-dyhydroxyvitamin D3 attenuates L-DOPA-induced neurotoxicity in neural stem cells. Mol Neurobiol 51:558–570
Restrepo-Angulo I, Bañuelos C, Camacho J (2020) Ion channel regulation by sex steroid hormones and vitamin D in cancer: a potential opportunity for cancer diagnosis and therapy. Front Pharmacol 11:152
Long W, Johnson J, Kalyaanamoorthy S, Light P (2023) TRPV1 channels as a newly identified target for vitamin D. Channels 15:360–374
Gooch H, Cui X, Anggono V, Trzaskowski M, Tan MC, Eyles DW et al (2019) 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Transl Psychiatry 9:281
Olszewska AM, Sieradzan AK, Bednarczyk P, Szewczyk A, Żmijewski MA (2022) Mitochondrial potassium channels: a novel calcitriol target. Cell Mol Biol Lett 27:3
Menegaz D, Dornelles CRT, Fern, Cavalari a C, Gonçalves R, Silva FRMB (2016) Potassium channels couple to 1,25(OH)2 vitamin D3 Rapid responses and secretion in immature sertoli cells. J Clin Mol Endocrinol 1:33
Zakon HH (1998) The effects of steroid hormones on electrical activity of excitable cells. Trends Neurosci 21:202–207
Kalueff AV, Tuohimaa P (2007) Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr Opin Clin Nutr Metab Care 10:12
Nadim F, Bucher D (2014) Neuromodulation of neurons and synapses. Curr Opin Neurobiol 29:48–56
Voglis G, Tavernarakis N (2006) The role of synaptic ion channels in synaptic plasticity. EMBO Rep 7:1104–1110
Chambon J, Komal P, Lewitus GM, Kemp GM, Valade S, Adaïdi H et al (2023) Early TNF-Dependent regulation of excitatory and inhibitory synapses on striatal direct pathway medium spiny neurons in the YAC128 mouse model of Huntington’s disease. J Neurosci Off J Soc Neurosci 43:672–680
Gáll Z, Székely O (2021) Role of vitamin D in cognitive dysfunction: new molecular concepts and discrepancies between animal and human findings. Nutrients 13:3672
Taghizadeh M, Talaei SA, Djazayeri A, Salami M (2014) Vitamin D supplementation restores suppressed synaptic plasticity in Alzheimer’s disease. Nutr Neurosci 17:172–177
Salami M, Talaei SA, Davari S, Taghizadeh M (2012) Hippocampal long term potentiation in rats under different regimens of vitamin D: an in vivo study. Neurosci Lett 509:56–59
Kouba BR, Torrá ACNC, Camargo A, Rodrigues ALS (2023) The antidepressant-like effect elicited by vitamin D3 is associated with BDNF/TrkB-related synaptic protein synthesis. Metab Brain Dis 38:601–611
Marsh J, Alifragis P (2018) Synaptic dysfunction in Alzheimer’s disease: the effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen Res 13:616–623
Taoufik E, Kouroupi G, Zygogianni O, Matsas R (2018) Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: an overview of induced pluripotent stem-cell-based disease models. Open Biol 8:180138
Baker K, Gordon SL, Melland H, Bumbak F, Scott DJ, Jiang TJ et al (2018) SYT1-associated neurodevelopmental disorder: a case series. Brain 141:2576–2591
Levy AM, Gomez-Puertas P, Tümer Z (2022) Neurodevelopmental disorders associated with PSD-95 and its interaction partners. Int J Mol Sci 23:4390
Mirza FJ, Zahid S (2017) The role of synapsins in neurological disorders. Neurosci Bull 34:349–358
Ando K, Ndjim M, Turbant S, Fontaine G, Pregoni G, Dauphinot L et al (2020) The lipid phosphatase Synaptojanin 1 undergoes a significant alteration in expression and solubility and is associated with brain lesions in Alzheimer’s disease. Acta Neuropathol Commun 8:79
Khassafi N, Zahraei Z, Vahidinia Z, Karimian M, Azami TA (2022) Calcitriol Pretreatment attenuates glutamate neurotoxicity by regulating NMDAR and CYP46A1 gene expression in rats subjected to transient middle cerebral artery occlusion. J Neuropathol Exp Neurol 81:252–259
Cass WA, Peters LE, Fletcher AM, Yurek DM (2012) Evoked dopamine overflow is augmented in the striatum of calcitriol treated rats. Neurochem Int 60:186–191
Orme RP, Bhangal MS, Fricker RA (2013) Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE 8:e62040
Trinko JR, Land BB, Solecki WB, Wickham RJ, Tellez LA, Maldonado-Aviles J et al (2016) Vitamin D3: a role in dopamine circuit regulation, diet-induced obesity, and drug consumption. eNeuro 3:0122–15
Simmons EC, Scholpa NE, Schnellmann RG (2020) Mitochondrial biogenesis as a therapeutic target for traumatic and neurodegenerative CNS diseases. Exp Neurol 329:113309
Jellinger KA (2010) Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med 14:457–487
Endres D, Werden R, Schweizer T, Schröter N, Schiele MA, Nickel K et al (2022) Novel neuronal autoantibodies in Huntington’s disease. Biol Psychiatry 91:e21–e23
Kocurova G, Ricny J, Ovsepian SV (2022) Autoantibodies targeting neuronal proteins as biomarkers for neurodegenerative diseases. Theranostics 12:3045–3056
Golde TE, Borchelt DR, Giasson BI, Lewis J (2013) Thinking laterally about neurodegenerative proteinopathies. J Clin Invest 123:1847–1855
Soto C, Pritzkow S (2018) Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci 21:1332–1340
Caron NS, Banos R, Aly AE, Xie Y, Ko S, Potluri N et al (2022) Cerebrospinal fluid mutant huntingtin is a biomarker for huntingtin lowering in the striatum of Huntington disease mice. Neurobiol Dis 166:105652
Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S et al (2011) Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J Alzheimers Dis JAD 25:295–307
Briones TL, Darwish H (2012) Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation 9:244
Patel P, Shah J (2017) Role of Vitamin D in Amyloid clearance via LRP-1 upregulation in Alzheimer’s disease: a potential therapeutic target? J Chem Neuroanat 85:36–42
Machiela E, Jeloka R, Caron NS, Mehta S, Schmidt ME, Baddeley HJE et al (2020) The interaction of aging and cellular stress contributes to pathogenesis in mouse and human Huntington disease neurons. Front Aging Neurosci 12:524369
Yuan A, Nixon RA (2021) Neurofilament proteins as biomarkers to monitor neurological diseases and the efficacy of therapies. Front Neurosci 15:689938
Liu Q, Xie F, Alvarado-Diaz A, Smith MA, Moreira PI, Zhu X et al (2011) Neurofilamentopathy in neurodegenerative diseases. Open Neurol J 5:58–62
Goischke H-K (2022) Neurofilament light chain determination: referee for future vitamin D3 supplementation in multiple sclerosis? NeuroImmunoModulation 29:520–522
Rodney C, Rodney S, Millis RM (2020) Vitamin D and demyelinating diseases: neuromyelitis optica (NMO) and multiple sclerosis (MS). Autoimmune Dis 2020:8718736
Corriveau RA, Huh GS, Shatz CJ (1998) Regulation of Class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21:505–520
Syken J, Shatz CJ (2003) Expression of T cell receptor beta locus in central nervous system neurons. Proc Natl Acad Sci U S A 100:13048–13053
Boulanger LM, Shatz CJ (2004) Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci 5:521–531
Goddard CA, Butts DA, Shatz CJ (2007) Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A 104:6828–6833
Boulanger LM (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64:93–109
Komal P, Nashmi R (2015) T-cell receptors modify neuronal function in the central nervous system. Biochem Pharmacol 97:512–517
Cebrián C, Loike JD, Sulzer D (2014) Neuronal MHC-I expression and its implications in synaptic function, axonal regeneration and Parkinson’s and other brain diseases. Front Neuroanat 8:114
Faust T, Gunner G, Schafer DP (2021) Mechanisms governing activity-dependent synaptic pruning in the mammalian CNS. Nat Rev Neurosci 22:657–673
Hong S, Dissing-Olesen L, Stevens B (2016) New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol 36:128–134
Cardozo PL, de Lima IBQ, Maciel EMA, Silva NC, Dobransky T, Ribeiro FM (2019) Synaptic elimination in neurological disorders. Curr Neuropharmacol 17:1071–1095
Sakai J (2020) Core Concept: How synaptic pruning shapes neural wiring during development and possibly, in disease. Proc Natl Acad Sci U S A 117:16096–16099
Wang B-Y, Ye Y-Y, Qian C, Zhang H-B, Mao H-X, Yao L-P et al (2021) Stress increases MHC-I expression in dopaminergic neurons and induces autoimmune activation in Parkinson’s disease. Neural Regen Res 16:2521–2527
Zalocusky KA, Najm R, Taubes AL, Hao Y, Yoon SY, Koutsodendris N et al (2021) Neuronal ApoE upregulates MHC-I expression to drive selective neurodegeneration in Alzheimer’s disease. Nat Neurosci 24:786–798
Staats K, Schonefeldt S, Van Rillaer M, Van Hoecke A, Van Damme P, Robberecht W et al. Beta-2 microglobulin is important for disease progression in a murine model for amyotrophic lateral sclerosis. Front Cell Neurosci. 2013;7.
Tetruashvily MM, McDonald MA, Frietze KK, Boulanger LM (2016) MHCI promotes developmental synapse elimination and aging-related synapse loss at the vertebrate neuromuscular junction. Brain Behav Immun 56:197–208
Zujovic V, Benavides J, Vigé X, Carter C, Taupin V (2000) Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia 29:305–315
Li M, Shang D-S, Zhao W-D, Tian L, Li B, Fang W-G et al (2009) Amyloid β interaction with receptor for advanced glycation end products up-regulates brain endothelial CCR5 expression and promotes T cells crossing the blood-brain barrier1. J Immunol 182:5778–5788
Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT (2010) Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68:19–31
Griffioen K, Mattson MP, Okun E (2018) Deficiency of Toll-like receptors 2, 3 or 4 extends life expectancy in Huntington’s disease mice. Heliyon 4:e00508
Zhang H, Lin A, Gong P, Chen Y, Ye RD, Qian F et al (2020) The chemokine-like receptor 1 deficiency improves cognitive deficits of AD mice and attenuates tau hyperphosphorylation via regulating tau seeding. J Neurosci 40:6991–7007
Hobson BD, Sulzer D (2022) Neuronal presentation of antigen and its possible role in Parkinson’s disease. J Park Dis 12:S137–S147
Wojcieszak J, Kuczyńska K, Zawilska JB (2022) Role of chemokines in the development and progression of Alzheimer’s disease. J Mol Neurosci 72:1929–1951
Zhong L, Sheng X, Wang W, Li Y, Zhuo R, Wang K et al (2023) TREM2 receptor protects against complement-mediated synaptic loss by binding to complement C1q during neurodegeneration. Immunity 56:1794-1808.e8
Lương Vinh Quốc K, Nguyễn LTH (2013) Roles of vitamin D in amyotrophic lateral sclerosis: possible genetic and cellular signaling mechanisms. Mol Brain 6:16
Moretti R, Morelli ME, Caruso P (2018) Vitamin D in neurological diseases: a rationale for a pathogenic impact. Int J Mol Sci 19:2245
Hochmeister S, Aeinehband S, Dorris C, Berglund R, Haindl MT, Velikic V et al (2020) Effect of vitamin D on experimental autoimmune neuroinflammation is dependent on haplotypes comprising naturally occurring allelic variants of ciiTA (Mhc2ta). Front Neurol 11:600401
Behl T, Arora A, Singla RK, Sehgal A, Makeen HA, Albratty M et al (2022) Understanding the role of “sunshine vitamin D” in Parkinson’s disease: a review. Front Pharmacol 13:993033
Janjusevic M, Gagno G, Fluca AL, Padoan L, Beltrami AP, Sinagra G et al (2022) The peculiar role of vitamin D in the pathophysiology of cardiovascular and neurodegenerative diseases. Life Sci 289:120193
Ghahremani M, Smith EE, Chen H-Y, Creese B, Goodarzi Z, Ismail Z (2023) Vitamin D supplementation and incident dementia: effects of sex, APOE, and baseline cognitive status. Alzheimers Dement Diagn Assess Dis Monit 15:e12404
Ruffo P, De Amicis F, Giardina E, Conforti FL (2022) Long-noncoding RNAs as epigenetic regulators in neurodegenerative diseases. Neural Regen Res 18:1243–1248
Park J, Lee K, Kim K, Yi S-J (2022) The role of histone modifications: from neurodevelopment to neurodiseases. Signal Transduct Target Ther 7:1–23
Lee AJ, Kim C, Park S et al (2023) Characterization of altered molecular mechanisms in Parkinson’s disease through cell type–resolved multiomics analyses. Sci Adv 9:2467
Ghafouri-Fard S, Khoshbakht T, Hussen BM et al (2022) The emerging role of long non-coding RNAs, microRNAs, and an accelerated epigenetic age in Huntington’s disease. Front Aging Neurosci. 14:987174
Hwang J-Y, Aromolaran KA, Zukin RS (2017) The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat Rev Neurosci 18:347–361
Pal S, Tyler JK (2016) Epigenetics and aging Sci Adv 2:e1600584
Rasmi Y, Shokati A, Hassan A et al (2022) The role of DNA methylation in progression of neurological disorders and neurodegenerative diseases as well as the prospect of using DNA methylation inhibitors as therapeutic agents for such disorders. IBRO Neurosci Rep 14:28–37
Kochmanski J, Kuhn NC, Bernstein AI (2022) Parkinson’s disease-associated, sex-specific changes in DNA methylation at PARK7 (DJ-1), SLC17A6 (VGLUT2), PTPRN2 (IA-2β), and NR4A2 (NURR1) in cortical neurons. Npj Park Dis 8:1–13
Sharma VK, Mehta V, Singh TG (2020) Alzheimer’s disorder: epigenetic connection and associated risk factors. Curr Neuropharmacol 18:740–753
Wheildon G, Weymouth LS, Smith AR et al (2022) DNA methylation profiling across brain regions in Huntington’s disease. Alzheimers Dement 18:e067590
Karlic H, Varga F (2011) Impact of vitamin D metabolism on clinical epigenetics. Clin Epigenetics 2:55–61
Xue J, Schoenrock SA, Valdar W et al (2016) Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin Epigenetics 8:107
Wimalawansa SJ (2019) Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. Biology 8:30
Fetahu IS, Höbaus J, Kállay E (2014) Vitamin D, and the epigenome. Front Physiol 5:164
Seuter S, Ryynänen J, Carlberg C (2014) The ASAP2 gene is a primary target of 1,25-dihydroxyvitamin D3 in human monocytes and macrophages. J Steroid Biochem Mol Biol 144(Pt A):12–18
Shukla S, Tekwani BL (2020) Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front Pharmacol 11:537
Li H, Shi G, Zha H et al (2021) Inhibition of histone deacetylase promotes a neuroprotective mechanism in an experimental model of Parkinson’s disease. Arch Med Sci 20:130287
Liu F-H, Li S-S, Li X-X et al (2017) Vitamin D3 induces vitamin D receptor and HDAC11 binding to relieve the promoter of the tight junction proteins. Oncotarget 8:58781–58789
Saidi L, Hammou H, Sicard F et al (2023) Maternal vitamin D deficiency and brain functions: a never-ending story. Food Funct 14:6290–6301
Pereira F, Barbáchano A, Silva J et al (2011) KDM6B/JMJD3 histone demethylase is induced by vitamin D and modulates its effects in colon cancer cells. Hum Mol Genet 20:4655–4665
Pereira F, Barbáchano A, Singh PK et al (2012) Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle Georget Tex 11:1081–1089
Baba R, Matsuda S, Maeda R et al (2022) Investigating the therapeutic potential of lsd1 enzyme activity-specific inhibition by TAK-418 for social and memory deficits in rodent disease models. ACS Chem Neurosci 13:313–321
Ambrosio S, Majello B (2018) Targeting histone demethylase LSD1/KDM1a in neurodegenerative diseases. J Exp Neurosci 12:1179069518765743
Mei Z, Hu H, Zou Y, Li D (2023) The role of Vitamin D in menopausal women’s health. Front Physiol 14:1211896
Pérez-López FR, Chedraui P, Pilz S (2020) Vitamin D supplementation after the menopause. Ther Adv Endocrinol Metab 11:2042018820931291
Xue Y, He X, Li H-D, Deng Y, Yan M, Cai H-L et al (2015) Simultaneous quantification of 25-hydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in rats shows strong correlations between serum and brain tissue levels. Int J Endocrinol 2015:296531
Bleizgys A (2021) Vitamin D dosing: basic principles and a brief algorithm (2021 Update). Nutrients 13:4415
Acknowledgements
We thank CSIR (Council of Scientific and Industrial Research) for MSKV’s SRF fellowship. MSKV, SAM, and OE are grateful for institutional fellowships provided by BITS-Pilani, Hyderabad. KG is obligated to DBT (BT/INF/22/SP42551/2021) for its research associate fellowship.
Funding
This work is supported by the SERB-SURE grant, Science and Engineering Research Board, Government of India (SUR/2022/000980), DBT builder grant, Department of Biotechnology (DBT; BT/INF/22/SP42551/2021), Government of India, the Young Maternity Parenthood grant award by the International Brain Research Organization (IBRO-2021).
Author information
Authors and Affiliations
Contributions
The manuscript and figures were prepared by MSKV, SAM, OE, KG, PJ, SM, and PK. The manuscript and figures were reviewed and edited by MSKV and PK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
SKV, M., Abraham, S.M., Eshwari, O. et al. Tremendous Fidelity of Vitamin D3 in Age-related Neurological Disorders. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03989-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03989-w