Abstract
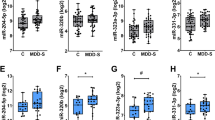
MicroRNAs (miRNAs) may contribute to the development of depression and its treatment. Here, we used the hypothesis-neutral approach of next-generation sequencing (NGS) to gain comprehensive understanding of the effects of a course of electroconvulsive stimulation (ECS), the animal model equivalent of electroconvulsive therapy (ECT), on rat hippocampal miRNAs. Significant differential expression (p < 0.001) of six hippocampal miRNAs was noted following NGS, after correcting for multiple comparisons. Three of these miRNAs were upregulated (miR-132, miR-212, miR-331) and three downregulated (miR-204, miR-483, miR-301a). qRT-PCR confirmed significant changes in four of the six miRNAs (miR-132, miR-212, miR-204, miR-483). miR-483 was also significantly reduced in frontal cortex, though no other significant alterations were noted in frontal cortex, cerebellum, or whole blood. Assessing the translatability of the results, miR-132 and miR-483 were significantly reduced in whole blood samples from medicated patients with depression (n = 50) compared to healthy controls (n = 45), though ECT had no impact on miRNA levels. Notably, pre-ECT miR-204 levels moderately positively correlated with depression severity at baseline and moderately negatively correlated with mood score reduction post-ECT. miRNAs were also examined in cerebrospinal fluid and serum from a separate cohort of patients (n = 8) treated with ECT; no significant changes were noted post-treatment. However, there was a large positive correlation between changes in miR-212 and mood score post-ECT in serum. Though replication studies using larger sample sizes are required, alterations in miRNA expression may be informative about the mechanism of action of ECS/ECT and in turn might give insight into the neurobiology of depression.



Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
UK Ect Review Group (2003) Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 361(9360):799–808. https://doi.org/10.1016/S0140-6736(03)12705-5
Kirov G, Jauhar S, Sienaert P, Kellner CH, McLoughlin DM (2021) Electroconvulsive therapy for depression: 80 years of progress. Brit J Psychiatry 219(5):594–597. https://doi.org/10.1192/bjp.2021.37
O’Donovan S, Kennedy M, Guinan B, O’Mara S, McLoughlin DM (2012) A comparison of brief pulse and ultrabrief pulse electroconvulsive stimulation on rodent brain and behaviour. Prog Neuropsychopharmacol Biol Psychiatry 37(1):147–152. https://doi.org/10.1016/j.pnpbp.2011.12.012
O’Donovan S, Dalton V, Harkin A, McLoughlin DM (2014) Effects of brief pulse and ultrabrief pulse electroconvulsive stimulation on rodent brain and behaviour in the corticosterone model of depression. Int J Neuropsychopharmacol 17(9):1477–1486. https://doi.org/10.1017/S1461145714000200
Sun W, Park KW, Choe J, Rhyu IJ, Kim IH, Park SK, Choi B, Choi SH et al (2005) Identification of novel electroconvulsive shock-induced and activity-dependent genes in the rat brain. Biochem Biophys Res Commun 327(3):848–856. https://doi.org/10.1016/j.bbrc.2004.12.050
Nibuya M, Morinobu S, Duman RS (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15(11):7539–7547
Newton SS, Girgenti MJ, Collier EF, Duman RS (2006) Electroconvulsive seizure increases adult hippocampal angiogenesis in rats. Eur J Neurosci 24(3):819–828. https://doi.org/10.1111/j.1460-9568.2006.04958.x
Elfving B, Bonefeld BE, Rosenberg R, Wegener G (2008) Differential expression of synaptic vesicle proteins after repeated electroconvulsive seizures in rat frontal cortex and hippocampus. Synapse 62(9):662–670. https://doi.org/10.1002/syn.20538
Olesen MV, Wortwein G, Folke J, Pakkenberg B (2017) Electroconvulsive stimulation results in long-term survival of newly generated hippocampal neurons in rats. Hippocampus 27(1):52–60. https://doi.org/10.1002/hipo.22670
Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, Danker-Hopfe H, Gass P (2009) Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 42(6):270–276. https://doi.org/10.1055/s-0029-1224162
Weber T, Baier V, Lentz K, Herrmann E, Krumm B, Sartorius A, Kronenberg G, Bartsch D (2013) Genetic fate mapping of type-1 stem cell-dependent increase in newborn hippocampal neurons after electroconvulsive seizures. Hippocampus 23(12):1321–1330. https://doi.org/10.1002/hipo.22171
Ryan KM, O’Donovan SM, McLoughlin DM (2013) Electroconvulsive stimulation alters levels of BDNF-associated microRNAs. Neurosci Lett 549:125–129. https://doi.org/10.1016/j.neulet.2013.05.035
O’Connor RM, Grenham S, Dinan TG, Cryan JF (2013) microRNAs as novel antidepressant targets: converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. Int J Neuropsychopharmacol 16(8):1885–1892. https://doi.org/10.1017/S1461145713000448
Kolshus E, Ryan KM, Blackshields G, Smyth P, Sheils O, McLoughlin DM (2017) Peripheral blood microRNA and VEGFA mRNA changes following electroconvulsive therapy: implications for psychotic depression. Acta Psychiatr Scand 136(6):594–606. https://doi.org/10.1111/acps.12821
Gururajan A, Naughton ME, Scott KA, O’Connor RM, Moloney G, Clarke G, Dowling J, Walsh A et al (2016) MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c. Transl Psychiatry 6(8):e862. https://doi.org/10.1038/tp.2016.131
Stavast CJ, Erkeland SJ (2019) The non-canonical aspects of microRNAs: many roads to gene regulation. Cells 8(11):1465. https://doi.org/10.3390/cells8111465
Kolshus E, Dalton VS, Ryan KM, McLoughlin DM (2014) When less is more–microRNAs and psychiatric disorders. Acta Psychiatr Scand 129(4):241–256. https://doi.org/10.1111/acps.12191
Chan AW, Kocerha J (2012) The path to microRNA therapeutics in psychiatric and neurodegenerative disorders. Front Genet 3:82. https://doi.org/10.3389/fgene.2012.00082
van den Berg MMJ, Krauskopf J, Ramaekers JG, Kleinjans JCS, Prickaerts J, Briede JJ (2020) Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog Neurobiol 185:101732. https://doi.org/10.1016/j.pneurobio.2019.101732
Xu YY, Xia QH, Xia QR, Zhang XL, Liang J (2019) MicroRNA-based biomarkers in the diagnosis and monitoring of therapeutic response in patients with depression. Neuropsychiatr Dis Treat 15:3583–3597. https://doi.org/10.2147/Ndt.S237116
Kuang WH, Dong ZQ, Tian LT, Li J (2018) MicroRNA-451a, microRNA-34a-5p, and microRNA-221-3p as predictors of response to antidepressant treatment. Braz J Med Biol Res 51(7):e7212. https://doi.org/10.1590/1414-431X20187212
Li YJ, Xu M, Gao ZH, Wang YQ, Yue Z, Zhang YX, Li XX, Zhang C et al (2013) Alterations of serum levels of BDNF-related miRNAs in patients with depression. PLoS ONE 8(5):e63648. https://doi.org/10.1371/journal.pone.0063648
Liu Y, Yang X, Zhao LS, Zhang J, Li T, Ma XH (2016) Increased miR-132 level is associated with visual memory dysfunction in patients with depression. Neuropsychiatr Dis Treat 12:2905–2911. https://doi.org/10.2147/Ndt.S116287
Roy B, Dunbar M, Shelton RC, Dwivedi Y (2017) Identification of microRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacol 42(4):864–875. https://doi.org/10.1038/npp.2016.175
Camkurt MA, Acar S, Coskun S, Gunes M, Gunes S, Yilmaz MF, Gorur A, Tamer L (2015) Comparison of plasma microRNA levels in drug naive, first episode depressed patients and healthy controls. J Psychiatric Res 69:67–71. https://doi.org/10.1016/j.jpsychires.2015.07.023
Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, Maussion G, Yang JP et al (2014) miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med 20(7):764–768. https://doi.org/10.1038/nm.3582
Liu W, Zhang F, Zheng Y, He S, Zhang T, Guo Q, Xu H, Chen H et al (2021) The role of circulating blood microRNA-374 and microRNA-10 levels in the pathogenesis and therapeutic mechanisms of major depressive disorder. Neurosci Lett 763:136184. https://doi.org/10.1016/j.neulet.2021.136184
Hung YY, Wu MK, Tsai MC, Huang YL, Kang HY (2019) Aberrant expression of intracellular let-7e, miR-146a, and miR-155 correlates with severity of depression in patients with major depressive disorder and is ameliorated after antidepressant treatment. Cells 8(7):647. https://doi.org/10.3390/cells8070647
Hung YY, Chou CK, Yang YC, Fu HC, Loh EW, Kang HY (2021) Exosomal let-7e, miR-21-5p, miR-145, miR-146a and miR-155 in predicting antidepressants response in patients with major depressive disorder. Biomedicines 9(10):1428. https://doi.org/10.3390/biomedicines9101428
Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Corrada D, Milanesi L, Gennarelli M (2013) Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol 23(7):602–611. https://doi.org/10.1016/j.euroneuro.2012.06.013
Lin CC, Tsai MC, Lee CT, Sun MH, Huang TL (2018) Antidepressant treatment increased serum miR-183 and miR-212 levels in patients with major depressive disorder. Psychiatry Res 270:232–237. https://doi.org/10.1016/j.psychres.2018.09.025
Fiori LM, Lopez JP, Richard-Devantoy S, Berlim M, Chachamovich E, Jollant F, Foster J, Rotzinger S et al (2017) Investigation of miR-1202, miR-135a, and miR-16 in major depressive disorder and antidepressant response. Int J Neuropsychopharmacol 20(8):619–623. https://doi.org/10.1093/ijnp/pyx034
Saeedi S, Nagy C, Ibrahim P, Théroux J-F, Wakid M, Fiori LM, Yang J, Rotzinger S et al (2021) Neuron-derived extracellular vesicles enriched from plasma show altered size and miRNA cargo as a function of antidepressant drug response. Mol Psychiatry 26:7417–7424. https://doi.org/10.1038/s41380-021-01255-2
Song W, Shen Y, Zhang Y, Peng S, Zhang R, Ning A, Li H, Li X et al (2019) Expression alteration of microRNAs in nucleus accumbens is associated with chronic stress and antidepressant treatment in rats. BMC Med Inform Decis Mak 19(Suppl 6):271. https://doi.org/10.1186/s12911-019-0964-z
Xin C, Xia J, Liu Y, Zhang Y (2020) MicroRNA-202-3p targets brain-derived neurotrophic factor and is involved in depression-like behaviors. Neuropsychiatr Dis Treat 16:1073–1083. https://doi.org/10.2147/NDT.S241136
Gu Z, Pan J, Chen L (2019) MiR-124 suppression in the prefrontal cortex reduces depression-like behavior in mice. Biosci Rep 39(9):BSR20190186. https://doi.org/10.1042/BSR20190186
Dai J, Pan JY, Liao N, Shi J, Zeng Q, Huang L, Chen LP (2020) Influence of miR-155 on behaviors of depression mice through regulating Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci 24(3):1398–1407. https://doi.org/10.26355/eurrev_202002_20197
Fang K, Xu JX, Chen XX, Gao XR, Huang LL, Du AQ, Jiang C, Ge JF (2020) Differential serum exosome microRNA profile in a stress-induced depression rat model. J Affect Disord 274:144–158. https://doi.org/10.1016/j.jad.2020.05.017
Huang C, Wang Y, Wu Z, Xu J, Zhou L, Wang D, Yang L, Zhu B et al (2021) miR-98-5p plays a critical role in depression and antidepressant effect of ketamine. Transl Psychiatry 11(1):454. https://doi.org/10.1038/s41398-021-01588-0
Miao N, Jin J, Kim SN, Sun T (2018) Hippocampal microRNAs respond to administration of antidepressant fluoxetine in adult mice. Int J Mol Sci 19(3):671. https://doi.org/10.3390/ijms19030671
First M, Spitzer R, Gibbon M, Williams J (1996) Structured clinical interview for DSM-IV axis I disorders, Clinician Version (SCID-CV). American Psychiatric Press, Washington DC
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Semkovska M, Landau S, Dunne R, Kolshus E, Kavanagh A, Jelovac A, Noone M, Carton M et al (2016) Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry 173(4):408–417. https://doi.org/10.1176/appi.ajp.2015.15030372
Ryan KM, Allers KA, McLoughlin DM, Harkin A (2020) Tryptophan metabolite concentrations in depressed patients before and after electroconvulsive therapy. Brain Behav Immun 83:153–162. https://doi.org/10.1016/j.bbi.2019.10.005
Mindt S, Neumaier M, Hellweg R, Sartorius A, Kranaster L (2020) Brain-derived neurotrophic factor in the cerebrospinal fluid increases during electroconvulsive therapy in patients with depression: a preliminary report. J ECT 36(3):193–197. https://doi.org/10.1097/YCT.0000000000000667
Ryan KM, McLoughlin DM (2019) Peripheral blood GILZ mRNA levels in depression and following electroconvulsive therapy. Psychoneuroendocrinol 101:304–310. https://doi.org/10.1016/j.psyneuen.2018.12.234
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 9:402. https://doi.org/10.3389/fendo.2018.00402
Oved K, Morag A, Pasmanik-Chor M, Oron-Karni V, Shomron N, Rehavi M, Stingl JC, Gurwitz D (2012) Genome-wide miRNA expression profiling of human lymphoblastoid cell lines identifies tentative SSRI antidepressant response biomarkers. Pharmacogenomics 13(10):1129–1139. https://doi.org/10.2217/pgs.12.93
Yi LT, Li J, Liu BB, Luo L, Liu Q, Geng D (2014) BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J Psychiatry Neurosci 39(5):348–359
Pan B, Liu Y (2015) Effects of duloxetine on microRNA expression profile in frontal lobe and hippocampus in a mouse model of depression. Int J Clin Exp Pathol 8(11):15454–15461
Hara Y, Ago Y, Takano E, Hasebe S, Nakazawa T, Hashimoto H, Matsuda T, Takuma K (2017) Prenatal exposure to valproic acid increases miR-132 levels in the mouse embryonic brain. Mol Autism 8:33. https://doi.org/10.1186/s13229-017-0149-5
Wang JQ, Mao L (2019) The ERK pathway: molecular mechanisms and treatment of depression. Mol Neurobiol 56(9):6197–6205. https://doi.org/10.1007/s12035-019-1524-3
Castren E, Monteggia LM (2021) Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry 90(2):128–136. https://doi.org/10.1016/j.biopsych.2021.05.008
Nestler EJ (2015) Role of the brain’s reward circuitry in depression: transcriptional mechanisms. Int Rev Neurobiol 124:151–170. https://doi.org/10.1016/bs.irn.2015.07.003
Si L, Wang Y, Liu M, Yang L, Zhang L (2021) Expression and role of microRNA-212/nuclear factor I-A in depressive mice. Bioengineered 12(2):11520–11532. https://doi.org/10.1080/21655979.2021.2009964
Cao MQ, Chen DH, Zhang CH, Wu ZZ (2013) Screening of specific microRNA in hippocampus of depression model rats and intervention effect of Chaihu Shugan San. Zhongguo Zhong Yao Za Zhi 38(10):1585–1589
Gawlinski D, Gawlinska K, Smaga I (2021) Maternal high-fat diet modulates Cnr1 gene expression in male rat offspring. Nutrients 13(8):2885. https://doi.org/10.3390/nu13082885
Lan T, Li Y, Fan C, Wang L, Wang W, Chen S, Yu SY (2021) MicroRNA-204-5p reduction in rat hippocampus contributes to stress-induced pathology via targeting RGS12 signaling pathway. J Neuroinflammation 18(1):243. https://doi.org/10.1186/s12974-021-02299-5
Gryglewski G, Lanzenberger R, Silberbauer LR, Pacher D, Kasper S, Rupprecht R, Frey R, Baldinger-Melich P (2021) Meta-analysis of brain structural changes after electroconvulsive therapy in depression. Brain Stimul 14(4):927–937. https://doi.org/10.1016/j.brs.2021.05.014
Deng ZD, Argyelan M, Miller J, Quinn DK, Lloyd M, Jones TR, Upston J, Erhardt E et al (2022) Electroconvulsive therapy, electric field, neuroplasticity, and clinical outcomes. Mol Psychiatry 27(3):1676–1682. https://doi.org/10.1038/s41380-021-01380-y
Lee WH, Deng ZD, Kim TS, Laine AF, Lisanby SH, Peterchev AV (2010) Regional electric field induced by electroconvulsive therapy: a finite element simulation study. Annu Int Conf IEEE Eng Med Biol Soc 2010:2045–2048. https://doi.org/10.1109/IEMBS.2010.5626553
Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR (2010) Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20(4):492–498. https://doi.org/10.1002/hipo.20646
Azevedo JA, Carter BS, Meng F, Turner DL, Dai M, Schatzberg AF, Barchas JD, Jones EG et al (2016) The microRNA network is altered in anterior cingulate cortex of patients with unipolar and bipolar depression. J Psychiatr Res 82:58–67. https://doi.org/10.1016/j.jpsychires.2016.07.012
Yu HC, Wu J, Zhang HX, Zhang GL, Sui J, Tong WW, Zhang XY, Nie LL et al (2015) Alterations of miR-132 are novel diagnostic biomarkers in peripheral blood of schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry 63:23–29. https://doi.org/10.1016/j.pnpbp.2015.05.007
Sun XY, Lu J, Zhang L, Song HT, Zhao L, Fan HM, Zhong AF, Niu W et al (2015) Aberrant microRNA expression in peripheral plasma and mononuclear cells as specific blood-based biomarkers in schizophrenia patients. J Clin Neurosci 22(3):570–574. https://doi.org/10.1016/j.jocn.2014.08.018
Fang Y, Qiu Q, Zhang S, Sun L, Li G, Xiao S, Li X (2018) Changes in miRNA-132 and miR-124 levels in non-treated and citalopram-treated patients with depression. J Affect Disord 227:745–751. https://doi.org/10.1016/j.jad.2017.11.090
Walker RM, Rybka J, Anderson SM, Torrance HS, Boxall R, Sussmann JE, Porteous DJ, McIntosh AM et al (2015) Preliminary investigation of miRNA expression in individuals at high familial risk of bipolar disorder. J Psychiatr Res 62:48–55. https://doi.org/10.1016/j.jpsychires.2015.01.006
Kim AH, Reimers M, Maher B, Williamson V, McMichael O, McClay JL, van den Oord EJ, Riley BP, Kendler KS, Vladimirov VI (2010) MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr Res 124(1–3):183–191. https://doi.org/10.1016/j.schres.2010.07.002
Weigelt K, Bergink V, Burgerhout KM, Pescatori M, Wijkhuijs A, Drexhage HA (2013) Down-regulation of inflammation-protective microRNAs 146a and 212 in monocytes of patients with postpartum psychosis. Brain Behav Immun 29:147–155. https://doi.org/10.1016/j.bbi.2012.12.018
Moghbeli M, Zangouei AS, Nasrpour Navaii Z, Taghehchian N (2021) Molecular mechanisms of the microRNA-132 during tumor progressions. Cancer Cell Int 21(1):439. https://doi.org/10.1186/s12935-021-02149-7
Ghafouri-Fard S, Hussen BM, Abak A, Taheri M, Jalili Khoshnoud R (2022) Aberrant expression of miRNAs in epilepsy. Mol Biol Rep 49(6):5057–5074. https://doi.org/10.1007/s11033-022-07188-5
Zhang M, Bian Z (2021) Alzheimer’s disease and microRNA-132: a widespread pathological factor and potential therapeutic target. Front Neurosci 15:687973. https://doi.org/10.3389/fnins.2021.687973
Yang Y, Li Y, Yang H, Guo J, Li N (2021) Circulating microRNAs and long non-coding RNAs as potential diagnostic biomarkers for Parkinson’s disease. Front Mol Neurosci 14:631553. https://doi.org/10.3389/fnmol.2021.631553
Yoshino Y, Roy B, Dwivedi Y (2021) Differential and unique patterns of synaptic miRNA expression in dorsolateral prefrontal cortex of depressed subjects. Neuropsychopharmacol 46(5):900–910. https://doi.org/10.1038/s41386-020-00861-y
Fiori LM, Kos A, Lin R, Theroux JF, Lopez JP, Kuhne C, Eggert C, Holzapfel M et al (2021) miR-323a regulates ERBB4 and is involved in depression. Mol Psychiatry 26(8):4191–4204. https://doi.org/10.1038/s41380-020-00953-7
Qi S, Yang X, Zhao L, Calhoun VD, Perrone-Bizzozero N, Liu S, Jiang R, Jiang T et al (2018) MicroRNA132 associated multimodal neuroimaging patterns in unmedicated major depressive disorder. Brain 141(3):916–926. https://doi.org/10.1093/brain/awx366
Sun XY, Zhang J, Niu W, Guo W, Song HT, Li HY, Fan HM, Zhao L et al (2015) A preliminary analysis of microRNA as potential clinical biomarker for schizophrenia. Am J Med Genet B Neuropsychiatr Genet 168B(3):170–178. https://doi.org/10.1002/ajmg.b.32292
Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, Willoughby D, Kenny PJ et al (2012) MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci USA 109(8):3125–3130. https://doi.org/10.1073/pnas.1113793109
Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ et al (2010) Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J 428(2):281–291. https://doi.org/10.1042/BJ20100024
Luikart BW, Bensen AL, Washburn EK, Perederiy JV, Su KG, Li Y, Kernie SG, Parada LF, Westbrook GL (2011) miR-132 mediates the integration of newborn neurons into the adult dentate gyrus. PLoS ONE 6(5):e19077. https://doi.org/10.1371/journal.pone.0019077
Aten S, Hansen KF, Hoyt KR, Obrietan K (2016) The miR-132/212 locus: a complex regulator of neuronal plasticity, gene expression and cognition. RNA Dis 3(2):e1375
Wanet A, Tacheny A, Arnould T, Renard P (2012) miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res 40(11):4742–4753. https://doi.org/10.1093/nar/gks151
Hansen KF, Sakamoto K, Aten S, Snider KH, Loeser J, Hesse AM, Page CE, Pelz C et al (2016) Targeted deletion of miR-132/-212 impairs memory and alters the hippocampal transcriptome. Learn Mem 23(2):61–71. https://doi.org/10.1101/lm.039578.115
Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH (2010) microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A 107(47):20382–20387. https://doi.org/10.1073/pnas.1015691107
Gruzdev SK, Yakovlev AA, Druzhkova TA, Guekht AB, Gulyaeva NV (2019) The missing link: how exosomes and miRNAs can help in bridging psychiatry and molecular biology in the context of depression, bipolar disorder and schizophrenia. Cell Mol Neurobiol 39(6):729–750. https://doi.org/10.1007/s10571-019-00684-6
An X, Wang Y (2022) Electroconvulsive shock increases neurotrophy and neurogenesis: time course and treatment session effects. Psychiatry Res 309:114390. https://doi.org/10.1016/j.psychres.2022.114390
Qian Y, Song J, Ouyang Y, Han Q, Chen W, Zhao X, Xie Y, Chen Y et al (2017) Advances in roles of miR-132 in the nervous system. Front Pharmacol 8:770. https://doi.org/10.3389/fphar.2017.00770
Xiang L, Ren Y, Li X, Zhao W, Song Y (2016) MicroRNA-204 suppresses epileptiform discharges through regulating TrkB-ERK1/2-CREB signaling in cultured hippocampal neurons. Brain Res 1639:99–107. https://doi.org/10.1016/j.brainres.2016.02.045
Lepko T, Pusch M, Muller T, Schulte D, Ehses J, Kiebler M, Hasler J, Huttner HB et al (2019) Choroid plexus-derived miR-204 regulates the number of quiescent neural stem cells in the adult brain. EMBO J 38(17):e100481. https://doi.org/10.15252/embj.2018100481
Liu H, Wang J, Yan R, Jin S, Wan Z, Cheng J, Li N, Chen L et al (2020) MicroRNA-204-5p mediates sevoflurane-induced cytotoxicity in HT22 cells by targeting brain-derived neurotrophic factor. Histol Histopathol 35(11):1353–1361. https://doi.org/10.14670/HH-18-266
Mohammed CP, Rhee H, Phee BK, Kim K, Kim HJ, Lee H, Park JH, Jung JH et al (2016) miR-204 downregulates EphB2 in aging mouse hippocampal neurons. Aging Cell 15(2):380–388. https://doi.org/10.1111/acel.12444
Liu H, Wang M, Xu L, Li M, Zhao M (2021) Neuroprotective effect of miR-204-5p downregulation against isoflurane-induced learning and memory impairment via targeting EphB2 and inhibiting neuroinflammation. Hum Exp Toxicol 40(10):1746–1754. https://doi.org/10.1177/09603271211009970
Pepe F, Visone R, Veronese A (2018) The glucose-regulated miR-483-3p influences key signaling pathways in cancer. Cancers (Basel) 10(6):181. https://doi.org/10.3390/cancers10060181
Luo YW, Xu Y, Cao WY, Zhong XL, Duan J, Wang XQ, Hu ZL, Li F et al (2015) Insulin-like growth factor 2 mitigates depressive behavior in a rat model of chronic stress. Neuropharmacol 89:318–324. https://doi.org/10.1016/j.neuropharm.2014.10.011
Bumb JM, Aksay SS, Janke C, Kranaster L, Geisel O, Gass P, Hellweg R, Sartorius A (2015) Focus on ECT seizure quality: serum BDNF as a peripheral biomarker in depressed patients. Eur Arch Psychiatry Clin Neurosci 265(3):227–232. https://doi.org/10.1007/s00406-014-0543-3
Ryan KM, McLoughlin DM (2018) From molecules to mind: mechanisms of action of electroconvulsive therapy. Acta Psychiatr Scand 138:177–179. https://doi.org/10.1111/acps.12951
Acknowledgements
The authors thank the patients and healthy volunteers who participated in this study.
Funding
This work was supported by a NARSAD Young Investigator Grant 2014 from the Brain and Behavior Research Foundation awarded to KR (22516) and an award from the Health Research Board (HRB), Ireland (TRA/2007/5&HPF/2010/17).
Author information
Authors and Affiliations
Contributions
KR, GB, PS, OS, and DM conceptualised and designed the study. KR, PM, and GB were involved in the acquisition and analysis of the data. LK and AS provided CSF and serum samples. KR and DM interpreted the data and drafted the manuscript. All authors critically reviewed the manuscript and approved its publication.
Corresponding author
Ethics declarations
Ethics Approval
Experimental procedures with animals were approved by the Bioresources Ethics Committee, Trinity College Dublin and were in compliance with the European Council Directive (86/609/EEC). Ethical approval for the human portion of this study was granted by the St Patrick’s University Hospital Research Ethics Committee in Dublin (Protocol No. 012/07) and the local ethics committee in Mannheim, Germany. The human studies were performed in accordance with the Declaration of Helsinki.
Consent to Participate
Written informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Competing Interests
DMM has received speaker’s honoraria from MECTA and Otsuka and an honorarium from Janssen for participating in an esketamine advisory board meeting. KR, LK, AS, PS, GB, and OS have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ryan, K.M., Smyth, P., Blackshields, G. et al. Electroconvulsive Stimulation in Rats Induces Alterations in the Hippocampal miRNome: Translational Implications for Depression. Mol Neurobiol 60, 1150–1163 (2023). https://doi.org/10.1007/s12035-022-03131-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03131-8