Abstract
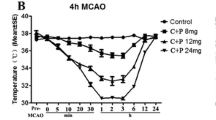
A depressive or hibernation-like effect of chlorpromazine and promethazine (C + P) on brain activity was reported to induce neuroprotection, with or without induced-hypothermia. However, the underlying mechanisms remain unclear. The current study evaluated the pharmacological function of C + P on the inhibition of neuroinflammatory response and inflammasome activation after ischemia/reperfusion. A total of 72 adult male Sprague–Dawley rats were subjected to 2 h middle cerebral artery occlusion (MCAO) followed by 6 or 24 h reperfusion. At the onset of reperfusion, rats received C + P (8 mg/kg) with temperature control. Brain cell death was detected by measuring CD68 and myeloperoxidase (MPO) levels. Inflammasome activation was measured by mRNA levels of NLRP3, IL-1β, and TXNIP, and protein quantities of NLRP3, IL-1β, TXNIP, cleaved-Caspase-1, and IL-18. Activation of JAK2/STAT3 pathway was detected by the phosphorylation of STAT3 (p-STAT3) and JAK2 (p-JAK2), and the co-localization of p-STAT3 and NLRP3. Activation of the p38 pathway was assessed with the protein levels of p-p38/p38. The mRNA and protein levels of HIF-1α, FoxO1, and p-FoxO1, and the co-localization of p-STAT3 with HIF-1α or FoxO1 were quantitated. As expected, C + P significantly reduced cell death and attenuated the neuroinflammatory response as determined by reduced CD68 and MPO. C + P decreased ischemia-induced inflammasome activation, shown by reduced mRNA and protein expressions of NLRP3, IL-1β, TXNIP, cleaved-Caspase-1, and IL-18. Phosphorylation of JAK2/STAT3 and p38 pathways and the co-localization of p-STAT3 with NLRP3 were also inhibited by C + P. Furthermore, mRNA levels of HIF-1α and FoxO1 were decreased in the C + P group. While C + P inhibited HIF-1α protein expression, it increased FoxO1 phosphorylation, which promoted the exclusion of FoxO1 from the nucleus and inhibited FoxO1 activity. At the same time, C + P reduced the co-localization of p-STAT3 with HIF-1α or FoxO1. In conclusion, C + P treatment conferred neuroprotection in stroke by suppressing neuroinflammation and NLRP3 inflammasome activation. The present study suggests that JAK2/STAT3/p38/HIF-1α/FoxO1 are vital regulators and potential targets for efficacious therapy following ischemic stroke.











Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Phipps MS, Cronin CA (2020) Management of acute ischemic stroke. BMJ 368:l6983. https://doi.org/10.1136/bmj.l6983
Ren C, Li N, Gao C, Zhang W, Yang Y, Li S, Ji X, Ding Y (2020) Ligustilide provides neuroprotection by promoting angiogenesis after cerebral ischemia. Neurol Res 42(8):683–692. https://doi.org/10.1080/01616412.2020.1782122
Fan X, Elkin K, Shi Y, Zhang Z, Cheng Y, Gu J, Liang J, Wang C, Ji X (2020) Schisandrin B improves cerebral ischemia and reduces reperfusion injury in rats through TLR4/NF-κB signaling pathway inhibition. Neurol Res 42(8):693–702. https://doi.org/10.1080/01616412.2020.1782079
González-Nieto D, Fernández-Serra R, Pérez-Rigueiro J, Panetsos F, Martinez-Murillo R, Guinea GV (2020) Biomaterials to neuroprotect the stroke brain: a large opportunity for narrow time windows. Cells 9(5):1074. https://doi.org/10.3390/cells9051074
Cotã CJ, Karl HW, Notterman DA, Weinberg JA, Mccloskey C (2000) Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics 106(4):633–644
Geng X, Li F, Yip J, Peng C, Ding Y (2017) Neuroprotection by chlorpromazine and promethazine in severe transient and permanent ischemic stroke. Mol Neurobiol 54(10):8140–8150. https://doi.org/10.1007/s12035-016-0280-x
Liu S, Geng X, Forreider B, Xiao Y, Kong Q, Ding Y, Ji X (2015) Enhanced beneficial effects of mild hypothermia by phenothiazine drugs in stroke therapy. Neurol Res 37(5):454–460. https://doi.org/10.1179/1743132815Y.0000000031
Guo S, Cosky E, Li F, Guan L, Ji Y, Wei W, Peng C, Geng X, Ding Y (2021) An inhibitory and beneficial effect of chlorpromazine and promethazine (C + P) on hyperglycolysis through HIF-1α regulation in ischemic stroke. Brain Res 1763:147463. https://doi.org/10.1016/j.brainres.2021.147463
Kuczynski AM, Demchuk AM, Almekhlafi MA (2019) Therapeutic hypothermia: applications in adults with acute ischemic stroke. Brain Circ 5(2):43–54. https://doi.org/10.4103/bc.bc_5_19
Wang J, Mao J, Wang R, Li S, Wu B, Yuan Y (2020) Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front Pharmacol 11:424–424. https://doi.org/10.3389/fphar.2020.00424
Guan L, Guo S, Yip J, Elkin KB, Li F, Peng C, Geng X, Ding Y (2019) Artificial hibernation by phenothiazines: a potential neuroprotective therapy against cerebral inflammation in stroke. Curr Neurovasc Res 16(3):232–240
Jiang Q, Wills M, Geng X, Ding Y (2021) Chlorpromazine and promethazine reduces brain injury through RIP1-RIP3 regulated activation of NLRP3 inflammasome following ischemic stroke. Neurol Res 43(8):668–676. https://doi.org/10.1080/01616412.2021.1910904
Donovan C, Liu G, Shen S, Marshall JE, Kim RY, Alemao CA, Budden KF, Choi JP, Kohonen-Corish M, El-Omar EM, Yang IA, Hansbro PM (2020) The role of the microbiome and the NLRP3 inflammasome in the gut and lung. J Leukoc Biol 108(3):925–935. https://doi.org/10.1002/jlb.3mr0720-472rr
An S, Hu H, Li Y, Hu Y (2020) Pyroptosis Plays a Role in Osteoarthritis. Aging Dis 11(5):1146–1157. https://doi.org/10.14336/AD.2019.1127
Wu J, Lin S, Wan B, Velani B, Zhu Y (2019) Pyroptosis in Liver Disease: New Insights into Disease Mechanisms. Aging Dis 10(5):1094–1108. https://doi.org/10.14336/AD.2019.0116
Davis BK, Wen H, Ting JP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29:707–735. https://doi.org/10.1146/annurev-immunol-031210-101405
Rathinam VA, Fitzgerald KA (2016) Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 165(4):792–800. https://doi.org/10.1016/j.cell.2016.03.046
Jiang Q, Geng X, Warren J, Eugene Paul Cosky E, Kaura S, Stone C, Li F, Ding Y (2020) Hypoxia Inducible Factor-1α (HIF-1α) Mediates NLRP3 Inflammasome-Dependent-Pyroptotic and Apoptotic Cell Death Following Ischemic Stroke. Neuroscience 448:126–139. https://doi.org/10.1016/j.neuroscience.2020.09.036
Dong Y, Hu C, Huang C, Gao J, Niu W, Wang D, Wang Y, Niu C (2021) Interleukin-22 Plays a Protective Role by Regulating the JAK2-STAT3 Pathway to Improve Inflammation, Oxidative Stress, and Neuronal Apoptosis following Cerebral Ischemia-Reperfusion Injury. Mediators Inflamm 2021:6621296. https://doi.org/10.1155/2021/6621296
Liang Z, Wu G, Fan C, Xu J, Jiang S, Yan X, Di S, Ma Z, Hu W, Yang Y (2016) The emerging role of signal transducer and activator of transcription 3 in cerebral ischemic and hemorrhagic stroke. Prog Neurobiol 137:1–16. https://doi.org/10.1016/j.pneurobio.2015.11.001
Chang L, Niu F, Chen J, Cao X, Liu Z, Bao X, Xu Y (2019) Ghrelin improves muscle function in dystrophin-deficient mdx mice by inhibiting NLRP3 inflammasome activation. Life Sci 232:116654. https://doi.org/10.1016/j.lfs.2019.116654
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL, Hu G (2012) Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses. PLoS ONE 7(11):e49701. https://doi.org/10.1371/journal.pone.0049701
Dong H, Cui B, Hao X (2019) MicroRNA-22 alleviates inflammation in ischemic stroke via p38 MAPK pathways. Mol Med Rep 20(1):735–744. https://doi.org/10.3892/mmr.2019.10269
Wang M, Liu Z, Hu S, Duan X, Zhang Y, Peng C, Peng D, Han L (2020) Taohong siwu decoction ameliorates ischemic stroke injury via suppressing pyroptosis. Front Pharmacol 11:590453. https://doi.org/10.3389/fphar.2020.590453
Chen SF, Pan MX, Tang JC, Cheng J, Zhao D, Zhang Y, Liao HB, Liu R, Zhuang Y, Zhang ZF, Chen J, Lei RX, Li SF, Li HT, Wang ZF, Wan Q (2020) Arginine is neuroprotective through suppressing HIF-1α/LDHA-mediated inflammatory response after cerebral ischemia/reperfusion injury. Mol Brain 13(1):63. https://doi.org/10.1186/s13041-020-00601-9
Wang L, Lu Y, Guan H, Jiang D, Guan Y, Zhang X, Nakano H, Zhou Y, Zhang Y, Yang L, Li H (2013) Tumor necrosis factor receptor-associated factor 5 is an essential mediator of ischemic brain infarction. J Neurochem 126(3):400–414. https://doi.org/10.1111/jnc.12207
Wu D, Zhi X, Duan Y, Zhang M, An H, Wei W, Dong K, Zhang Y, Shi J, He X, Zhang J, Wu C, Meng R, Ding Y, Ji X (2019) Inflammatory cytokines are involved in dihydrocapsaicin (DHC) and regional cooling infusion (RCI)-induced neuroprotection in ischemic rat. Brain Res 1710:173–180. https://doi.org/10.1016/j.brainres.2018.12.033
Li F, Geng X, Yip J, Ding Y (2019) Therapeutic target and cell-signal communication of chlorpromazine and promethazine in attenuating blood-brain barrier disruption after ischemic stroke. Cell Transplant 28(2):145–156. https://doi.org/10.1177/0963689718819443
Geng X, Fu P, Ji X, Peng C, Fredrickson V, Sy C, Meng R, Ling F et al (2013) Synergetic neuroprotection of normobaric oxygenation and ethanol in ischemic stroke through improved oxidative mechanism. Stroke 44(5):1418–1425. https://doi.org/10.1161/STROKEAHA.111.000315
Almekhlafi M, Poli S, Goyal M, Demchuk A (2019) Therapeutic hypothermia in stroke: Quo Vadis? Brain Circ 5(4):157–159. https://doi.org/10.4103/bc.bc_62_19
Pollmächer T, Haack M, Schuld A, Kraus T, Hinze-Selch D (2000) Effects of antipsychotic drugs on cytokine networks. J Psychiatr Res 34(6):369–382. https://doi.org/10.1016/s0022-3956(00)00032-7
Basta-Kaim A, Budziszewska B, Jaworska-Feil L, Tetich M, Kubera M, Zajicova A, Holan V, Lasoń W (2004) Effects of lipopolysaccharide and chlorpromazine on glucocorticoid receptor-mediated gene transcription and immunoreactivity: a possible involvement of p38-MAP kinase. Eur Neuropsychopharmacol 14(6):521–528. https://doi.org/10.1016/j.euroneuro.2004.02.005
Masuda K, Kishimoto T (2018) A Potential Therapeutic Target RNA-binding Protein, Arid5a for the Treatment of Inflammatory Disease Associated with Aberrant Cytokine Expression. Curr Pharm Des 24(16):1766–1771. https://doi.org/10.2174/1381612824666180426103753
Tong Y, Elkin KB, Peng C, Shen J, Li F, Guan L, Ji Y, Wei W et al (2019) Reduced apoptotic injury by phenothiazine in ischemic stroke through the NOX-Akt/PKC pathway. Brain Sci 9(12):378. https://doi.org/10.3390/brainsci9120378
Zeng Z, Zhang Y, Liang X, Wang F, Zhao J, Xu Z, Liu X, Liu X (2019) Qingnao dripping pills mediate immune-inflammatory response and MAPK signaling pathway after acute ischemic stroke in rats. J Pharmacol Sci 139(3):143–150. https://doi.org/10.1016/j.jphs.2018.12.009
Chen S, Chen H, Du Q, Shen J (2020) Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front Physiol 11:433. https://doi.org/10.3389/fphys.2020.00433
Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, Sarau HM, Clark RK, Griswold DE (1991) Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res 29(3):336–345. https://doi.org/10.1002/jnr.490290309
Cojocaru IM, Cojocaru M, Iliescu I, Botnaru L, Gurban CV, Sfrijan F, Tănăsescu R (2010) Plasma myeloperoxidase levels in patients with acute ischemic stroke. Rom J Intern Med 48(1):101–104
Rice RA, Pham J, Lee RJ, Najafi AR, West BL, Green KN (2017) Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 65(6):931–944. https://doi.org/10.1002/glia.23135
Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD et al (2006) The cellular inflammatory response in human spinal cords after injury. Brain 129(Pt 12):3249–3269. https://doi.org/10.1093/brain/awl296
Liu X, Wu Z, Hayashi Y, Nakanishi H (2012) Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience 216:133–142. https://doi.org/10.1016/j.neuroscience.2012.04.050
Duris K, Splichal Z, Jurajda M (2018) The Role of Inflammatory Response in Stroke Associated Programmed Cell Death. Curr Neuropharmacol 16(9):1365–1374. https://doi.org/10.2174/1570159x16666180222155833
Hou Y, Wang Y, He Q, Li L, Xie H, Zhao Y, Zhao J (2018) Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav Brain Res 336:32–39. https://doi.org/10.1016/j.bbr.2017.06.027
Tegowski M, Fan C, Baldwin AS (2018) Thioridazine inhibits self-renewal in breast cancer cells via DRD2-dependent STAT3 inhibition, but induces a G(1) arrest independent of DRD2. J Biol Chem 293(41):15977–15990. https://doi.org/10.1074/jbc.RA118.003719
Tsai C, Ikematsu K, Sakai S, Matsuo A, Nakasono I (2011) Expression of Bcl2l1, Clcf 1, IL-28ra and Pias1 in the mouse heart after single and repeated administration of chlorpromazine. Leg Med (Tokyo) 13(5):221–225. https://doi.org/10.1016/j.legalmed.2011.04.006
Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, Dournaud P, Gressens P et al (2013) The role of JAK-STAT signaling within the CNS. JAKSTAT 2(1):e22925. https://doi.org/10.4161/jkst.22925
Li L, Sun L, Qiu Y, Zhu W, Hu K, Mao J (2020) Protective Effect of Stachydrine Against Cerebral Ischemia-Reperfusion Injury by Reducing Inflammation and Apoptosis Through P65 and JAK2/STAT3 Signaling Pathway. Front Pharmacol 11:64–64. https://doi.org/10.3389/fphar.2020.00064
Sui Y, Bian L, Ai Q, Yao Y, Yu M, Gao H, Zhang A, Fu X, Zhong L, Lu D (2019) Gastrodin Inhibits Inflammasome Through the STAT3 Signal Pathways in TNA2 Astrocytes and Reactive Astrocytes in Experimentally Induced Cerebral Ischemia in Rats. NeuroMol Med 21(3):275–286. https://doi.org/10.1007/s12017-019-08544-8
Liu CC, Huang ZX, Li X, Shen KF, Liu M, Ouyang HD, Zhang SB, Ruan YT, Zhang XL, Wu SL, Xin WJ, Ma C (2018) Upregulation of NLRP3 via STAT3-dependent histone acetylation contributes to painful neuropathy induced by bortezomib. Exp Neurol 302:104–111. https://doi.org/10.1016/j.expneurol.2018.01.011
Cheng H, Lv M, Mi R, Xue G (2020) Amifostine ameliorates cerebral ischaemia-reperfusion injury via p38-mediated oxidative stress and mitochondrial dysfunction. Folia Neuropathol 58(4):334–346. https://doi.org/10.5114/fn.2020.102436
Li D, Ren W, Jiang Z, Zhu L (2018) Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol Med Rep 18(5):4399–4409. https://doi.org/10.3892/mmr.2018.9427
Bracken CP, Fedele AO, Linke S, Balrak W, Lisy K, Whitelaw ML, Peet DJ (2006) Cell-specific regulation of hypoxiainducible factor (HIF)-1alpha and HIF-2alpha stabilization and transactivation in a graded oxygen environment. J Biol Chem 281(32):22575–22585. https://doi.org/10.1074/jbc.M600288200
Talwar H, Bauerfeld C, Bouhamdan M, Farshi P, Liu Y, Samavati L (2017) MKP-1 negatively regulates LPS-mediated IL-1β production through p38 activation and HIF-1α expression. Cell Signal 34:1–10. https://doi.org/10.1016/j.cellsig.2017.02.018
Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S et al (2005) STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J 19(10):1296–1298. https://doi.org/10.1096/fj.04-3099fje
Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE (2008) Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci 11(4):476–487. https://doi.org/10.1038/nn2071
Li Y, Xiang L, Wang C, Song Y, Miao J, Miao M (2021) Protection against acute cerebral ischemia/reperfusion injury by Leonuri Herba Total Alkali via modulation of BDNF-TrKB-PI3K/Akt signaling pathway in rats. Biomed Pharmacother 133:111021. https://doi.org/10.1016/j.biopha.2020.111021
Li W, Zhu Q, Xu X, Hu X (2021) MiR-27a-3p suppresses cerebral ischemia-reperfusion injury by targeting FOXO1. Aging (Albany NY) 13(8):11727–11737. https://doi.org/10.18632/aging.202866
Nyandwi J, Ko Y, Jin H, Yun S, Park S, Kim H (2020) Rosmarinic acid inhibits oxLDL-induced inflammasome activation under high-glucose conditions through downregulating the p38-FOXO1-TXNIP pathway. Biochem Pharmacol 182:114246. https://doi.org/10.1016/j.bcp.2020.114246
Li X, Kover KL, Heruth DP, Watkins DJ, Moore WV, Jackson K, Zang M, Clements MA et al (2015) New insight into metformin action: Regulation of ChREBP and FOXO1 activities in endothelial cells. Mol Endocrinol 29(8):1184–1194. https://doi.org/10.1210/me.2015-1090
Zeng R, Luo D, Li H, Zhang Q, Lei S, Chen J (2019) MicroRNA-135b alleviates MPP-mediated Parkinson’s disease in in vitro model through suppressing FoxO1-induced NLRP3 inflammasome and pyroptosis. J Clin Neurosci 65:125–133. https://doi.org/10.1016/j.jocn.2019.04.004
Kim D, Kim S, Lee B, Lee E, Chung K, Moon K, An H, Kim K, Yu B, Chung H (2017) Effect of betaine on hepatic insulin resistance through FOXO1-induced NLRP3 inflammasome. J Nutr Biochem 45:104–114. https://doi.org/10.1016/j.jnutbio.2017.04.014
Kortylewski M, Feld F, Krüger K, Bahrenberg G, Roth R, Joost H, Heinrich P, Behrmann I, Barthel A (2003) Akt modulates STAT3-mediated gene expression through a FKHR (FOXO1a)-dependent mechanism. J Biol Chem 278(7):5242–5249. https://doi.org/10.1074/jbc.M205403200
Sun W, Wang B, Qu XL, Zheng BQ, Huang WD, Sun ZW, Wang CM, Chen Y (2019) Metabolism of reactive oxygen species in osteosarcoma and potential treatment applications. Cells 9(1):87. https://doi.org/10.3390/cells9010087
Raeis V, Philip-Couderc P, Roatti A, Habre W, Sierra J, Kalangos A, Beghetti M, Baertschi AJ (2010) Central venous hypoxemia is a determinant of human atrial ATP-sensitive potassium channel expression: evidence for a novel hypoxia-inducible factor 1alpha-Forkhead box class O signaling pathway. Hypertension 55(5):1186–1192. https://doi.org/10.1161/HYPERTENSIONAHA.109.148767
Funding
This work was supported in part by the National Natural Science Foundation of China (81871838, 82001277, 82101436), the Beijing Tongzhou District Financial Fund (2021), the Laboratory Development Funds of Luhe Hospital (2021).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yuchuan Ding, Xiaokun Geng and Sichao Guo.; Methodology: Sichao Guo; Formal analysis and investigation: Sichao Guo; Writing—original draft preparation: Sichao Guo; Writing—review and editing: Yuchuan Ding, Xiaokun Geng and Hangil Lee; Funding acquisition: Xiaokun Geng; Resources: Yuchuan Ding and Xiaokun Geng; Supervision: Yuchuan Ding and Xiaokun Geng.
Corresponding authors
Ethics declarations
Ethics Approval
Animal experiments were approved by the Institutional Animal Investigation Committee of the Capital Medical University and were performed in accordance with the Guidelines for Animal Experiments at Capital Medical University.
Consent to Participate
No human subjects were involved in this research, so consent to participate is not relevant.
Consent for Publication
All authors have approved this manuscript and consented to its submission for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, S., Geng, X., Lee, H. et al. Phenothiazine Inhibits Neuroinflammation and Inflammasome Activation Independent of Hypothermia After Ischemic Stroke. Mol Neurobiol 58, 6136–6152 (2021). https://doi.org/10.1007/s12035-021-02542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02542-3