Abstract
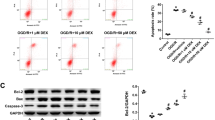
Angiotensin II receptor blockers (ARBs) have been shown to exert neuroprotective effects by suppressing inflammatory and apoptotic responses. In the present study, the effects of the ARB telmisartan on the NLRP3 inflammasome induced by oxygen-glucose deprivation (OGD) in neural stem cells (NSCs) were investigated, as well as their possible association with the activation of the PI3K pathway. Cultured NSCs were treated with different concentrations of telmisartan and subjected to various durations of OGD. Cell counting, lactate dehydrogenase, bromodeoxyuridine, and colony-forming unit assays were performed to measure cell viability and proliferation. In addition, the activity of intracellular signaling pathways associated with the PI3K pathway and NLRP3 inflammasome was evaluated. Telmisartan alone did not affect NSCs up to a concentration of 10 μM under normal conditions but showed toxicity at a concentration of 100 μM. Moreover, OGD reduced the viability of NSCs in a time-dependent manner. Nevertheless, treatment with telmisartan increased the viability and proliferation of OGD-injured NSCs. Furthermore, telmisartan promoted the expression of survival-related proteins and mRNA while inhibiting the expression of death-related proteins induced by OGD. In particular, telmisartan attenuated OGD-dependent expression of the NLRP3 inflammasome and its related signaling proteins. These beneficial effects of telmisartan were blocked by a PI3K inhibitor. Together, these results indicate that telmisartan attenuated the activation of the NLRP3 inflammasome by triggering the PI3K pathway, thereby contributing to neuroprotection.








Similar content being viewed by others
References
Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL et al (2014) Global and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. Lancet 383(9913):245–254. https://doi.org/10.1016/s0140-6736(13)61953-4
Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J (2000) Generalized potential of adult neural stem cells. Science 288(5471):1660–1663. https://doi.org/10.1126/science.288.5471.1660
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY (2010) A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28(6):1099–1106. https://doi.org/10.1002/stem.430
Koh SH, Park HH (2017) Neurogenesis in stroke recovery. Transl Stroke Res 8(1):3–13. https://doi.org/10.1007/s12975-016-0460-z
Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: Molecular activation and regulation of therapeutics. Nat Rev Immunol 19(8):477–489. https://doi.org/10.1038/s41577-019-0165-0
Gao L, Dong Q, Song Z, Shen F, Shi J, Li Y (2017) NLRP3 inflammasome: A promising target in ischemic stroke. Inflamm Res 66(1):17–24. https://doi.org/10.1007/s00011-016-0981-7
Koh SH, Lo EH (2015) The role of the PI3K pathway in the regeneration of the damaged brain by neural stem cells after cerebral infarction. J Clin Neurol 11(4):297–304. https://doi.org/10.3988/jcn.2015.11.4.297
Hou Y, Wang K, Wan W, Cheng Y, Pu X, Ye X (2018) Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis 5(3):245–255. https://doi.org/10.1016/j.gendis.2018.06.001
Wang C, Wei Z, Jiang G, Liu H (2017) Neuroprotective mechanisms of miR-124 activating the PI3K/Akt signaling pathway in ischemic stroke. Exp Ther Med 13(6):3315–3318. https://doi.org/10.3892/etm.2017.4424
Saavedra JM (2012) Angiotensin II AT(1) receptor blockers ameliorate inflammatory stress: A beneficial effect for the treatment of brain disorders. Cell Mol Neurobiol 32(5):667–681. https://doi.org/10.1007/s10571-011-9754-6
Villapol S, Saavedra JM (2015) Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens 28(3):289–299. https://doi.org/10.1093/ajh/hpu197
Saavedra JM (2017) Beneficial effects of angiotensin II receptor blockers in brain disorders. Pharmacol Res 125(Pt A):91–103. https://doi.org/10.1016/j.phrs.2017.06.017
Choi NY, Kim JY, Hwang M, Lee EH, Choi H, Lee KY, Lee YJ, Koh SH (2019) Atorvastatin rejuvenates neural stem cells injured by oxygen-glucose deprivation and induces neuronal differentiation by activating the PI3K/Akt and ERK pathways. Mol Neurobiol 56(4):2964–2977. https://doi.org/10.1007/s12035-018-1267-6
Holloway PM, Gavins FN (2016) Modeling ischemic stroke in vitro: Status quo and future perspectives. Stroke 47(2):561–569. https://doi.org/10.1161/strokeaha.115.011932
Wang J, Pang T, Hafko R, Benicky J, Sanchez-Lemus E, Saavedra JM (2014) Telmisartan ameliorates glutamate-induced neurotoxicity: Roles of AT1 receptor blockade and PPARγ activation. Neuropharmacology 79:249–261. https://doi.org/10.1016/j.neuropharm.2013.11.022
Khallaf WAI, Messiha BAS, Abo-Youssef AMH, El-Sayed NS (2017) Protective effects of telmisartan and tempol on lipopolysaccharide-induced cognitive impairment, neuroinflammation, and amyloidogenesis: Possible role of brain-derived neurotrophic factor. Can J Physiol Pharmacol 95(7):850–860. https://doi.org/10.1139/cjpp-2017-0042
Saravanan PB, Shanmuganathan MV, Ramanathan M (2015) Telmisartan attenuated LPS-induced neuroinflammation in a human IMR-32 neuronal cell line via SARM in an AT1R independent mechanism. Life Sci 130:88–96. https://doi.org/10.1016/j.lfs.2015.03.005
Kono S, Kurata T, Sato K, Omote Y, Hishikawa N, Yamashita T, Deguchi K, Abe K (2015) Neurovascular protection by telmisartan via reducing neuroinflammation in stroke-resistant spontaneously hypertensive rat brain after ischemic stroke. J Stroke Cerebrovasc Dis 24(3):537–547. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.09.037
Zhuo X, Wu Y, Yang Y, Gao L, Qiao X, Chen T (2019) Knockdown of LSD1 meliorates Ox-LDL-stimulated NLRP3 activation and inflammation by promoting autophagy via the SESN2-mesiated PI3K/Akt/mTOR signaling pathway. Life Sci 233:116696. https://doi.org/10.1016/j.lfs.2019.116696
Lee YC, Kim SR, Lee KB, Park HJ, Jeong JS (2015) NLRP3 inflammasome is activated via the phosphoinositide 3-kinase δ pathway in Aspergillus fumigatus-induced allergic airway inflammation. Eur Respir J 46(suppl 59):PA1889. https://doi.org/10.1183/13993003.congress-2015.PA1889
Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM (2007) ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282(5):2871–2879. https://doi.org/10.1074/jbc.M608083200
Chan EWL, Krishnansamy S, Wong C, Gan SY (2019) The NLRP3 inflammasome is involved in the neuroprotective mechanism of neural stem cells against microglia-mediated toxicity in SH-SY5Y cells via the attenuation of tau hyperphosphorylation and amyloidogenesis. Neurotoxicology 70:91–98. https://doi.org/10.1016/j.neuro.2018.11.001
Kaushal V, Dye R, Pakavathkumar P, Foveau B, Flores J, Hyman B, Ghetti B, Koller BH et al (2015) Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ 22(10):1676–1686. https://doi.org/10.1038/cdd.2015.16
Ji J, Xiang P, Li T, Lan L, Xu X, Lu G, Ji H, Zhang Y et al (2017) NOSH-NBP, a novel nitric oxide and hydrogen sulfide- releasing hybrid, attenuates ischemic stroke-induced neuroinflammatory injury by modulating microglia polarization. Front Cell Neurosci 11:154. https://doi.org/10.3389/fncel.2017.00154
Shi F, Yang L, Kouadir M, Yang Y, Wang J, Zhou X, Yin X, Zhao D (2012) The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J Neuroinflammation 9:73. https://doi.org/10.1186/1742-2094-9-73
Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert JM, Raussens V (2015) Amyloid fibrils are molecular triggers of inflammation in Parkinson’s disease. Biochem J 471(3):323–333. https://doi.org/10.1042/bj20150617
Yu JW, Lee MS (2016) Mitochondria and the NLRP3 inflammasome: Physiological and pathological relevance. Arch Pharm Res 39(11):1503–1518. https://doi.org/10.1007/s12272-016-0827-4
Park HH, Yu HJ, Kim S, Kim G, Choi NY, Lee EH, Lee YJ, Yoon MY et al (2016) Neural stem cells injured by oxidative stress can be rejuvenated by GV1001, a novel peptide, by scavenging free radicals and enhancing survival signals. Neurotoxicology 55:131–141. https://doi.org/10.1016/j.neuro.2016.05.022
Saavedra JM, Sanchez-Lemus E, Benicky J (2011) Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation, and ischemia: Therapeutic implications. Psychoneuroendocrinology 36(1):1–18. https://doi.org/10.1016/j.psyneuen.2010.10.001
Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J et al (2004) Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activity. Hypertension 43(5):993–1002. https://doi.org/10.1161/01.hyp.0000123072.34629.57
Miao H, Ou J, Ma Y, Guo F, Yang Z, Wiggins M, Liu C, Song W et al (2014) Macrophage CGI-58 deficiency activates the ROS-inflammasome pathway to promote insulin resistance in mice. Cell Rep 7(1):223–235. https://doi.org/10.1016/j.celrep.2014.02.047
Fuentes-Antrás J, Ioan AM, Tuñón J, Egido J, Lorenzo O (2014) Activation of toll-like receptors and inflammasome complexes in diabetic cardiomyopathy-associated inflammation. Int J Endocrinol 10:847827. https://doi.org/10.1155/2014/847827
Pinar AA, Scott TE, Huuskes BM, Tapia Cáceres FE, Kemp-Harper BK, Samuel CS (2020) Targeting the NLRP3 inflammasome to treat cardiovascular fibrosis. Pharmacol Ther 209:107511. https://doi.org/10.1016/j.pharmthera.2020.107511
Pang T, Sun LX, Wang T, Jiang ZZ, Liao H, Zhang LY (2014) Telmisartan protects central neurons against nutrient deprivation-induced apoptosis in vitro through activation of PPARγ and the Akt/GSK-3β pathway. Acta Pharmacol Sin 35(6):727–737. https://doi.org/10.1038/aps.2013.199
Marx N, Duez H, Fruchart JC, Staels B (2004) Peroxisome proliferator-activated receptors and atherogenesis: Regulators of gene expression in vascular cells. Circ Res 94(9):1168–1178. https://doi.org/10.1161/01.res.0000127122.22685.0a
Li X, Bilali A, Qiao R, Paerhati T, Yang Y (2018) Association of the PPARγ/PI3K/Akt pathway with the cardioprotective effects of tacrolimus in myocardial ischemic/reperfusion injury. Mol Med Rep 17(5):6759–6767. https://doi.org/10.3892/mmr.2018.8649
Stravodimou A, Mazzoccoli G, Voutsadakis IA (2012) Peroxisome proliferator-activated receptor gamma and regulation by the ubiquitin-proteasome system in pancreatic cancer. PPAR Res 13:367450. https://doi.org/10.1155/2012/367450
Lv S, Wang W, Wang H, Zhu Y, Lei C (2019) PPARγ activation serves as a therapeutic strategy against bladder cancer by inhibiting the PI3K-Akt signaling pathway. BMC Cancer 19(1):204. https://doi.org/10.1186/s12885-019-5426-6
Zhang P, Zhang Y, Chen X, Li R, Yin J, Zhong D (2006) Pharmacokinetics of telmisartan in healthy Chinese subjects after oral administration of two dosage levels. Arzneimittelforschung 56(8):569–573. https://doi.org/10.1055/s-0031-1296753
De Filippis L, Binda E (2012) Concise review: Self-renewal in the central nervous system: Neural stem cells from embryo to adult. Stem Cells Transl Med 1(4):298–308. https://doi.org/10.5966/sctm.2011-0045
Lee J, Park HH, Koh SH, Choi H (2017) Neural stem cell death mechanisms induced by amyloid beta. Dement Neurocogn dis 16(4):121–127. https://doi.org/10.12779/dnd.2017.16.4.121
Chojnacki A, Weiss S (2008) Production of neurons, astrocytes, and oligodendrocytes from mammalian CNS stem cells. Nat Protoc 3(6):935–940. https://doi.org/10.1038/nprot.2008.55
Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, Kumar S (2003) CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci 116(Pt 13):2677–2685. https://doi.org/10.1242/jcs.00470
Park HH, Han MH, Choi H, Lee YJ, Kim JM, Cheong JH, Ryu JI, Lee KY et al (2019) Mitochondria damaged by oxygen glucose deprivation can be restored through activation of the PI3K/Akt pathway and inhibition of calcium influx by amlodipine camsylate. Sci Rep 9(1):15717. https://doi.org/10.1038/s41598-019-52083-y
Acknowledgments
We thank all members of Prof. Koh’s Lab for scientific advice and helps.
Availability of Data and Material
Not applicable.
Funding
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea, which is funded by the Ministry of Science, ICT, and Future Planning (2018R1A2A2A15023219); by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI18C1254); and by the Medical Research Center (2017R1A5A2015395).
Author information
Authors and Affiliations
Contributions
The study conception and design: K.H.S, H.J, and K.S-H. Investigation: H.J., P.H.H, and L.E-H. Data analysis and interpretation of data: K.H.S and HJ. Supervision: K.J.Y., C.H., L.K-Y., and L.Y.J. Original draft: K.H.S and H.J. Revised manuscript: K.J.Y. and K.S-H. K.H.S and H.J. contributed equally to this work.
Corresponding author
Ethics declarations
All animal procedures were conducted in accordance with Hanyang University’s guidelines for the care and use of laboratory animals, and approved by the Institutional Animal Care and Use Committee (IACUC) of Hanyang University (2019-0162A). All efforts were made to minimize the number of animals used and animal suffering.
Consent for Publication
All authors have approved the manuscript and agree with its publication.
Consent to Participate
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Suppl. Fig. 1
Identification of NSCs via immunocytochemistry (a) and RT-PCR (b). Scale bars for immunocytochemistry = 50 μm. RT-PCR analysis: Gapdh, 112 bp; nestin, 234 bp; Sox2, 414 bp. (PNG 629 kb)
Suppl. Fig. 2
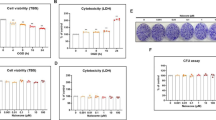
Effects of telmisartan and OGD on the viability and cytotoxicity of NSCs. Cell viability (a, trypan blue staining) was significantly decreased and cytotoxicity (b, LDH assay) significantly increased in a time-dependent manner after exposure to OGD. Telmisartan treatment alone up to a concentration of 10 μM did not affect the viability (c) and cytotoxicity (d) of NSCs. Data are presented as mean (% of control) ± SD. Treatment groups were compared with the control group using one-way ANOVA followed by Tukey’s test (n = 4). *P < 0.05, **P < 0.01 (vs. the control group); #P < 0.05, ##P < 0.01 (vs. the group only subjected to an 8-h OGD). (PNG 384 kb)
Suppl. Fig. 3
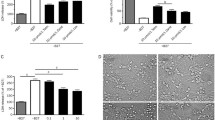
Photographs of NSCs showing the effects of telmisartan on the viability of NSCs injured by OGD. NSCs were photographed after exposure to OGD at different time points (a), after telmisartan treatment at various concentrations (b), and after both an 8-h exposure to OGD and telmisartan treatment at various concentrations (c). (PNG 2454 kb)
Suppl. Fig. 4
Photographs of NSCs showing the effects of telmisartan on ROS generation via detection of DCF by fluorescent microscopy. (PNG 1271 kb)
Suppl. Fig. 5
Effects of GW9662 on the viability and cytotoxicity of NSCs. GW9662 treatment up to a concentration of 10 μM for 24 h did not affect the viability (a, trypan blue staining) or cytotoxicity (b, LDH assay) of NSCs (a: n = 4 and b: n = 3). (PNG 115 kb)
Rights and permissions
About this article
Cite this article
Kwon, H.S., Ha, J., Kim, J.Y. et al. Telmisartan Inhibits the NLRP3 Inflammasome by Activating the PI3K Pathway in Neural Stem Cells Injured by Oxygen-Glucose Deprivation. Mol Neurobiol 58, 1806–1818 (2021). https://doi.org/10.1007/s12035-020-02253-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02253-1