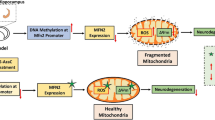
Abstract
The recurrent events of mild trauma exacerbate the vulnerability for post-traumatic stress disorder; however, the underlying molecular mechanisms are scarcely known. The repeated mild traumatic brain injury (rMTBI) perturbs redox homeostasis which is primarily managed by superoxide dismutase 2 (SOD2). The current study investigates the role of DNA methylation in SOD2 gene regulation and its involvement in rMTBI-induced persistent neuropathology inflicted by weight drop injury paradigm. The oxidative damage, neurodegenerative indicators, and SOD2 function and its regulation in the hippocampus were analyzed after 48 h and 30 days of rMTBI. The temporal and episodic increase in ROS levels (oxidative stress) heightened 8-hydroxyguanosine levels indicating oxidative damage after rMTBI that was concomitant with decline in SOD2 function. In parallel, occupancy of DNMT3b at SOD2 promoter was higher post 30 days of the first episode of rMTBI causing hypermethylation at SOD2 promoter. This epigenetic silencing of SOD2 promoter was sustained after the second episode of rMTBI causing permanent blockade in SOD2 response. The resultant oxidative stress further culminated into the increasing number of degenerating neurons. The treatment with 5-azacytidine, a pan DNMT inhibitor, normalized DNA methylation levels and revived SOD2 function after the second episode of rMTBI. The release of blockade in SOD2 expression by DNMT inhibition also normalized the post-traumatic oxidative consequences and relieved the neurodegeneration and deficits in learning and memory as measured by novel object recognition test. In conclusion, DNMT3b-mediated DNA methylation plays a critical role in SOD2 gene regulation in the hippocampus, and the perturbations therein post rMTBI are detrimental to redox homeostasis manifesting into neurological consequences.







Similar content being viewed by others
Change history
11 November 2020
Supplementary Information has been changed by a cleaned file.
References
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO et al (2019) Estimating the global incidence of traumatic brain injury. J Neurosurg 130:1080–1097. https://doi.org/10.3171/2017.10.JNS17352
Theadom A, Starkey NJ, Dowell T, Hume PA, Kahan M, McPherson K, Feigin V, BIONIC Research Group (2014) Sports-related brain injury in the general population: an epidemiological study. J Sci Med Sport 17:591–596. https://doi.org/10.1016/j.jsams.2014.02.001
Manley G, Gardner AJ, Schneider KJ, Guskiewicz KM, Bailes J, Cantu RC, Castellani RJ, Turner M et al (2017) A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med 51:969–977. https://doi.org/10.1136/bjsports-2017-097791
Peskind ER, Brody D, Cernak I, McKee A, Ruff RL (2013) Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J Clin Psychiatry 74:180–188. https://doi.org/10.4088/JCP.12011co1c
McKee AC, Robinson ME (2014) Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement 10. https://doi.org/10.1016/j.jalz.2014.04.003
Belanger HG, Spiegel E, Vanderploeg RD (2010) Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. J Int Neuropsychol Soc 16:262–267. https://doi.org/10.1017/S1355617709991287
Combs HL, Berry DTR, Pape T, Babcock-Parziale J, Smith B, Schleenbaker R, Shandera-Ochsner A, Harp JP et al (2015) The effects of mild traumatic brain injury, post-traumatic stress disorder, and combined mild traumatic brain injury/post-traumatic stress disorder on returning veterans. J Neurotrauma 32:956–966. https://doi.org/10.1089/neu.2014.3585
Vasterling JJ, Brailey K, Proctor SP, Kane R, Heeren T, Franz M (2012) Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in Iraq-deployed US Army soldiers. Br J Psychiatry 201:186–192. https://doi.org/10.1192/bjp.bp.111.096461
Ling H, Morris HR, Neal JW, Lees AJ, Hardy J, Holton JL, Revesz T, Williams DDR (2017) Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 133:337–352. https://doi.org/10.1007/s00401-017-1680-3
Shively SB, Edgerton SL, Iacono D, Purohit DP, Qu BX, Haroutunian V, Davis KL, Diaz-Arrastia R et al (2017) Localized cortical chronic traumatic encephalopathy pathology after single, severe axonal injury in human brain. Acta Neuropathol 133:353–366. https://doi.org/10.1007/s00401-016-1649-7
Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP (2012) Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma 29:281–294. https://doi.org/10.1089/neu.2011.2123
Sagarkar S, Bhamburkar T, Shelkar G, Choudhary A, Kokare DM, Sakharkar AJ (2017) Minimal traumatic brain injury causes persistent changes in DNA methylation at BDNF gene promoters in rat amygdala: a possible role in anxiety-like behaviors. Neurobiol Dis 106:101–109. https://doi.org/10.1016/j.nbd.2017.06.016
Sagarkar S, Balasubramanian N, Mishra S, Choudhary AG, Kokare DM, Sakharkar AJ (2019) Repeated mild traumatic brain injury causes persistent changes in histone deacetylase function in hippocampus: implications in learning and memory deficits in rats. Brain Res 1711:183–192. https://doi.org/10.1016/j.brainres.2019.01.022
Prins ML, Alexander D, Giza CC, Hovda DA (2013) Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J Neurotrauma 30:30–38. https://doi.org/10.1089/neu.2012.2399
Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI (2014) Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab 34:1223–1232. https://doi.org/10.1038/jcbfm.2014.75
Luo J, Nguyen A, Villeda S, Zhang H, Ding Z, Lindsey D, Bieri G, Castellano JM et al (2014) Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front Neurol 5:12. https://doi.org/10.3389/fneur.2014.00012
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99:4–9. https://doi.org/10.1093/bja/aem131
Bains M, Hall ED (2012) Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta Mol basis Dis 1822:675–684. https://doi.org/10.1016/j.bbadis.2011.10.017
Cornelius C, Crupi R, Calabrese V, Graziano A, Milone P, Pennisi G, Radak Z, Calabrese EJ et al (2013) Traumatic brain injury: oxidative stress and neuroprotection. Antioxid Redox Signal 19:836–853. https://doi.org/10.1089/ars.2012.4981
Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7:728–741. https://doi.org/10.1016/S1474-4422(08)70164-9
Rodriguez-Rodriguez A, Egea-Guerrero J, Murillo-Cabezas F, Carrillo-Vico A (2014) Oxidative stress in traumatic brain injury. Curr Med Chem 21:1201–1211. https://doi.org/10.2174/0929867321666131217153310
Arundine M, Tymianski M (2004) Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci 61:657–668. https://doi.org/10.1007/s00018-003-3319-x
Abdul-Muneer PM, Chandra N, Haorah J (2015) Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51:966–979. https://doi.org/10.1007/s12035-014-8752-3
Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED (2006) Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab 26:1407–1418. https://doi.org/10.1038/sj.jcbfm.9600297
Flynn JM, Melovn S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62:4–12. https://doi.org/10.1016/j.freeradbiomed.2013.05.027
Carvajal FJ, Mira RG, Rovegno M, Minniti AN, Cerpa W (2018) Age-related NMDA signaling alterations in SOD2 deficient mice. Biochim Biophys Acta Mol basis Dis 1864:2010–2020. https://doi.org/10.1016/j.bbadis.2018.03.019
Oh SS, Sullivan KA, Wilkinson JE, Backus C, Hayes JM, Sakowski SA, Feldman EL (2012) Neurodegeneration and early lethality in superoxide dismutase 2-deficient mice: a comprehensive analysis of the central and peripheral nervous systems. Neuroscience 212:201–213. https://doi.org/10.1016/j.neuroscience.2012.03.026
Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL (2008) A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging 29:117–128. https://doi.org/10.1016/j.neurobiolaging.2006.09.014
Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL (2007) SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol 208:216–227. https://doi.org/10.1016/j.expneurol.2007.07.017
Namjoshi DR, Good C, Cheng WH, Panenka W, Richards D, Cripton PA, Wellington CL (2013) Towards clinical management of traumatic brain injury: a review of models and mechanisms from a biomechanical perspective. Dis Model Mech 6:1325–1338. https://doi.org/10.1242/dmm.011320
Mychasiuk R, Farran A, Angoa-Perez M, Briggs D, Kuhn D, Esser MJ (2014) A novel model of mild traumatic brain injury for juvenile rats. J Vis Exp 94. https://doi.org/10.3791/51820
Xiong Y, Shie FS, Zhang J, Lee CP, Ho YS (2005) Prevention of mitochondrial dysfunction in post-traumatic mouse brain by superoxide dismutase. J Neurochem 95:732–744. https://doi.org/10.1111/j.1471-4159.2005.03412.x
Khatri N, Thakur M, Pareek V, Kumar S, Sharma S, Datusalia AK (2018) Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets 17:689–695. https://doi.org/10.2174/1871527317666180627120501
Huber BR, Meabon JS, Martin TJ, Mourad PD, Bennett R, Kraemer BC, Cernak I, Petrie EC et al (2013) Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J Alzheimers Dis 37:309–323. https://doi.org/10.3233/JAD-130182
Kuehner JN, Bruggeman EC, Wen Z, Yao B (2019) Epigenetic regulations in neuropsychiatric disorders. Front Genet 10. https://doi.org/10.3389/fgene.2019.00268
Stricker SH, Götz M (2018) DNA-methylation: master or slave of neural fate decisions? Front Neurosci 12:2018. https://doi.org/10.3389/fnins.2018.00005.eCollection
Haghighi F, Ge Y, Chen S, Xin Y, Umali MU, de Gasperi R, Gama Sosa MA, Ahlers ST et al (2015) Neuronal DNA methylation profiling of blast-related traumatic brain injury. J Neurotrauma 32:1200–1209. https://doi.org/10.1089/neu.2014.3640
Jia J, Zhang L, Shi X, Wu M, Zhou X, Liu X, Huo T (2016) SOD2 mediates amifostine-induced protection against glutamate in PC12 cells. Oxidative Med Cell Longev 2016. https://doi.org/10.1155/2016/4202437
Bharne AP, Borkar CD, Bodakuntla S, Lahiri M, Subhedar NK, Kokare DM (2016) Pro-cognitive action of CART is mediated via ERK in the hippocampus. Hippocampus 26:1313–1327. https://doi.org/10.1002/hipo.22608
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31:47–59. https://doi.org/10.1016/0166-4328(88)90157-X
Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA (2010) Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci 124:559–573. https://doi.org/10.1037/a0020893
Aggleton JP, Albasser MM, Aggleton DJ, Poirier GL, Pearce JM (2010) Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behav Neurosci 124:55–68. https://doi.org/10.1037/a0018320
Oliveira AMM, Hawk JD, Abel T, Havekes R (2010) Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 17:155–160. https://doi.org/10.1101/lm.1625310
Jaillard T, Roger M, Galinier A, Guillou P, Benani A, Leloup C, Casteilla L, Penicaud L et al (2009) Hypothalamic reactive oxygen species are required for insulin-induced food intake inhibition: an NADPH oxidase-dependent mechanism. Diabetes 58:1544–1549. https://doi.org/10.2337/db08-1039
Sagarkar S, Mahajan S, Choudhary AG, Borkar CD, Kokare DM, Sakharkar AJ (2017) Traumatic stress-induced persistent changes in DNA methylation regulate neuropeptide Y expression in rat jejunum. Neurogastroenterol Motil 29. https://doi.org/10.1111/nmo.13074
Balasubramanian N, Srivastava A, Pawar N, Sagarkar S, Sakharkar AJ (2019) Repeated mild traumatic brain injury induces persistent variations in mitochondrial DNA copy number in mesocorticolimbic neurocircuitry of the rat. Neurosci Res 155:34–42. https://doi.org/10.1016/j.neures.2019.06.003
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Thorvaldsdóttir H, Robinson JT, Mesirov JP (2013) Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14:178–192. https://doi.org/10.1093/bib/bbs017
Ehara A, Ueda S (2009) Application of Fluoro-Jade C in acute and chronic neurodegeneration models: utilities and staining differences. Acta Histochem Cytochem 42:171–179. https://doi.org/10.1267/ahc.09018
Lovell MA, Markesbery WR (2001) Ratio of 8-hydroxyguanine in intact DNA to free 8-hydroxyguanine is increased in Alzheimer disease ventricular cerebrospinal fluid. Arch Neurol 58:392–396. https://doi.org/10.1001/archneur.58.3.392
Massaad CA, Washington TM, Pautler RG, Klann E (2009) Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 106:13576–13581. https://doi.org/10.1073/pnas.0902714106
Ansari MA, Roberts KN, Scheff SW (2008) Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med 45:443–452. https://doi.org/10.1016/j.freeradbiomed.2008.04.038
Yang LY, Greig NH, Tweedie D, Jung YJ, Chiang YH, Hoffer BJ, Miller JP, Chang KH et al (2020) The p53 inactivators pifithrin-μ and pifithrin-α mitigate TBI-induced neuronal damage through regulation of oxidative stress, neuroinflammation, autophagy and mitophagy. Exp Neurol 324. https://doi.org/10.1016/j.expneurol.2019.113135
Huang YN, Yang LY, Greig NH, Wang YC, Lai CC, Wang JY (2018) Neuroprotective effects of pifithrin-α against traumatic brain injury in the striatum through suppression of neuroinflammation, oxidative stress, autophagy, and apoptosis. Sci Rep 8:2368. https://doi.org/10.1038/s41598-018-19654-x
Krishnamurthy K, Laskowitz DT (2016) Cellular and molecular mechanisms of secondary neuronal injury following traumatic brain injury. In: Translational Research in Traumatic Brain Injury. pp. 97–126
Weitzman SA, Turk PW, Milkowski DH, Kozlowski K (1994) Free radical adducts induce alterations in DNA cytosine methylation. Proc Natl Acad Sci U S A 91:1261–1264. https://doi.org/10.1073/pnas.91.4.1261
Fan W, Luo J (2010) SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol Cell 39:247–258. https://doi.org/10.1016/j.molcel.2010.07.006
Zhao H, Sun P, Fan T, Yang X, Zheng T, Sun C (2019) The effect of glutamate-induced excitotoxicity on DNA methylation in astrocytes in a new in vitro neuron-astrocyte-endothelium co-culture system. Biochem Biophys Res Commun 508:1209–1214. https://doi.org/10.1016/j.bbrc.2018.12.058
Bailey ZS, Grinter MB, De La Torre CD, VandeVord PJ (2015) Blast induced neurotrauma causes overpressure dependent changes to the DNA methylation equilibrium. Neurosci Lett 604:119–123. https://doi.org/10.1016/j.neulet.2015.07.035
Zhang ZY, Zhang Z, Fauser U, Schluesener HJ (2007) Global hypomethylation defines a sub-population of reactive microglia/macrophages in experimental traumatic brain injury. Neurosci Lett 429:1–6. https://doi.org/10.1016/j.neulet.2007.09.061
Griñán-Ferré C, Sarroca S, Ivanova A, Puigoriol-Illamola D, Aguado F, Camins A, Sanfeliu C, Pallàs M (2016) Epigenetic mechanisms underlying cognitive impairment and Alzheimer disease hallmarks in 5XFAD mice. Aging (Albany NY) 8:664–684. https://doi.org/10.18632/aging.100906
Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X et al (2012) Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci U S A 109:2515–2520. https://doi.org/10.1073/pnas.1120600109
Parabucki AB, Božić ID, Bjelobaba IM, Lavrnja IC, Brkić PD, Jovanović TS, Savić DZ, Stojiljković MB et al (2012) Hyperbaric oxygenation alters temporal expression pattern of superoxide dismutase 2 after cortical stab injury in rats. Croat Med J 53:586–597. https://doi.org/10.3325/cmj.2012.53.586
Iverson GL (2005) Outcome from mild traumatic brain injury. Curr Opin Psychiatry 18:301–317. https://doi.org/10.1097/01.yco.0000165601.29047.ae
Barkhoudarian G, Hovda DA, Giza CC (2011) The molecular pathophysiology of concussive brain injury. Clin Sports Med 30:33–48. https://doi.org/10.1016/j.csm.2010.09.001
Giza CC, Hovda DA (2014) The new neurometabolic cascade of concussion. Neurosurgery 75:S24–S33. https://doi.org/10.1227/NEU.0000000000000505
Liang LP, Waldbaum S, Rowley S, Huang TT, Day BJ, Patel M (2012) Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: Attenuation by a lipophilic metalloporphyrin. Neurobiol Dis 45:1068–1076. https://doi.org/10.1016/j.nbd.2011.12.025
Cowan K, Anichtchik O, Luo S (2019) Mitochondrial integrity in neurodegeneration. CNS Neurosci Ther 25:825–836
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94:909–950
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta, Mol Cell Res 1863:2977–2992. https://doi.org/10.1016/j.bbamcr.2016.09.012
Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, de Marchi E, Missiroli S et al (2012) Mitochondria-Ros crosstalk in the control of cell death and aging. J Signal Transduct 2012:1–17. https://doi.org/10.1155/2012/329635
Schaffert J, LoBue C, White CL, Chiang HS, Didehbani N, Lacritz L, Rossetti H, Dieppa M et al (2018) Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy-confirmed Alzheimer’s disease. Neuropsychology 32:410–416. https://doi.org/10.1037/neu0000423
Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, Tian G (2017) Head injury as a risk factor for dementia and Alzheimer’s disease: a systematic review and meta-analysis of 32 observational studies. PLoS One 12:e0169650. https://doi.org/10.1371/journal.pone.0169650
Abner EL, Nelson PT, Schmitt FA, Browning SR, Fardo DW, Wan L, Jicha GA, Cooper GE et al (2014) Self-reported head injury and risk of late-life impairment and AD pathology in an AD center cohort. Dement Geriatr Cogn Disord 37:294–306. https://doi.org/10.1159/000355478
Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74:857–862. https://doi.org/10.1136/jnnp.74.7.857
Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, Yaffe K (2018) Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology 90:E1771–E1779. https://doi.org/10.1212/WNL.0000000000005522
Raj R, Kaprio J, Korja M, Mikkonen ED, Jousilahti P, Siironen J (2017) Risk of hospitalization with neurodegenerative disease after moderate-to-severe traumatic brain injury in the working-age population: a retrospective cohort study using the Finnish national health registries. PLoS Med 14:e1002316. https://doi.org/10.1371/journal.pmed.1002316
Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, Sonnen J, Montine TJ et al (2016) Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 73:1062–1069. https://doi.org/10.1001/jamaneurol.2016.1948
Jafari S, Etminan M, Aminzadeh F, Samii A (2013) Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 28:1222–1229. https://doi.org/10.1002/mds.25458
Fernandes MYD, Carmo MRSD, Fonteles AA, Neves JCS, Silva ATAD, Pereira JF, Ferreira EO, Lima NMR et al (2019) (-)-Α-Bisabolol prevents neuronal damage and memory deficits through reduction of proinflammatory markers induced by permanent focal cerebral ischemia in mice. Eur J Pharmacol 842:270–280. https://doi.org/10.1016/j.ejphar.2018.09.036
Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, Giacovazzo G, Krashia P, Rizzo FR et al (2017) Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun 8. https://doi.org/10.1038/ncomms14727
Kotloski R, Lynch M, Lauersdorf S, Sutula T (2002) Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. In: Progress in Brain Research. pp. 95–110
Lee B, Sur B, Cho SG, Yeom M, Shim I, Lee H, Hahm DH (2016) Wogonin attenuates hippocampal neuronal loss and cognitive dysfunction in trimethyltin-intoxicated rats. Biomol Ther 24:328–337. https://doi.org/10.4062/biomolther.2015.152
Min D, Mao X, Wu K, Cao Y, Guo F, Zhu S, Xie N, Wang L et al (2012) Donepezil attenuates hippocampal neuronal damage and cognitive deficits after global cerebral ischemia in gerbils. Neurosci Lett 510:29–33. https://doi.org/10.1016/j.neulet.2011.12.064
Mouzon B, Chaytow H, Crynen G, Bachmeier C, Stewart J, Mullan M, Stewart W, Crawford F (2012) Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J Neurotrauma 29:2761–2773. https://doi.org/10.1089/neu.2012.2498
Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ (2013) History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front Aging Neurosci 5. https://doi.org/10.3389/fnagi.2013.00041
Yan H, Feng Y, Wang Q (2016) Altered effective connectivity of hippocampus-dependent episodic memory network in mTBI survivors. Neural Plast 2016:1–12. https://doi.org/10.1155/2016/6353845
Brueckner B, Boy RG, Siedlecki P, Musch T, Kliem HC, Zielenkiewicz P, Suhai S, Wiessler M (2005) Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res 65:6305–6311. https://doi.org/10.1158/0008-5472.CAN-04-2957
Gayet O, Loncle C, Duconseil P, Gilabert M, Lopez MB, Moutardier V, Turrini O, Calvo E et al (2015) A subgroup of pancreatic adenocarcinoma is sensitive to the 5-aza-dC DNA methyltransferase inhibitor. Oncotarget 6:746–754. https://doi.org/10.18632/oncotarget.2685
Yang PM, Lin YT, Shun CT, Lin SH, Wei TT, Chuang SH, Wu MS, Chen CC (2013) Zebularine inhibits tumorigenesis and stemness of colorectal cancer via p53-dependent endoplasmic reticulum stress. Sci Rep 3. https://doi.org/10.1038/srep03219
Endres M, Meisel A, Biniszkiewicz D, Namura S, Prass K, Ruscher K, Lipski A, Jaenisch R et al (2000) DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci 20:3175–3181. https://doi.org/10.1523/jneurosci.20-09-03175.2000
Kondo N, Tohnai G, Sahashi K, Iida M, Kataoka M, Nakatsuji H, Tsutsumi Y, Hashizume A et al (2019) DNA methylation inhibitor attenuates polyglutamine-induced neurodegeneration by regulating Hes5. EMBO Mol Med 11. https://doi.org/10.15252/emmm.201708547
Gaignard P, Fréchou M, Liere P, Thérond P, Schumacher M, Slama A, Guennoun R (2018) Sex differences in brain mitochondrial metabolism: influence of endogenous steroids and stroke. J Neuroendocrinol 30. https://doi.org/10.1111/jne.12497
Khalifa ARM, Abdel-Rahman EA, Mahmoud AM, Ali MH, Noureldin M, Saber SH, Mohsen M, Ali SS (2017) Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol Rep 5. https://doi.org/10.14814/phy2.13125
Demarest TG, McCarthy MM (2014) Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J Bioenerg Biomembr 47:173–188
Rubin TG, Lipton ML (2019) Sex differences in animal models of traumatic brain injury. J Exp Neurosci 13:117906951984402. https://doi.org/10.1177/1179069519844020
Gupte R, Brooks W, Vukas R, Pierce J, Harris J (2019) Sex differences in traumatic brain injury: what we know and what we should know. J Neurotrauma 36:3063–3091
Acknowledgments
The authors kindly acknowledge Dr. Chaitanya Athale, Indian Institute of Science Education and Research-Pune (IISER-Pune), India, for the generous gift of PC12 cell line cultures and Institute for Applied Biological Research and Development (IABRD), Pune, for their technical support in in vitro experiments.
Funding
This work was supported by the grants from the University Grants Commission, Government of India (UGC-GOI; F.4-5/151-FRP/2014/BSR); Science and Engineering Research Board (SERB), GOI (EMR/2017/000621); and Council for Scientific and Industrial Research (CSIR), GOI (37[1718]/18/EMR-II) to AJS. AJS also acknowledges funds received through the Department Research and Development Program of the Department of Biotechnology, Savitribai Phule Pune University, Pune, India. NB thanks the UGC-GOI for the award of Senior Research Fellowship (File No. 2061330923). SS acknowledges DST INSPIRE-Faculty program (DST/INSPIRE/04/2018/000529) for the funding support. DMK acknowledges the grants from the University Research Project Scheme (URPS; Dev./RTMNURP/AH/2115) from Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 350 kb).
Rights and permissions
About this article
Cite this article
Balasubramanian, N., Sagarkar, S., Choudhary, A.G. et al. Epigenetic Blockade of Hippocampal SOD2 Via DNMT3b-Mediated DNA Methylation: Implications in Mild Traumatic Brain Injury-Induced Persistent Oxidative Damage. Mol Neurobiol 58, 1162–1184 (2021). https://doi.org/10.1007/s12035-020-02166-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02166-z