Abstract
Deposition of an amyloid-β peptide is one of the first events in the pathophysiology of Alzheimer’s disease (AD) and is clinically characterized by Aβ plaques, tau tangles, and behavioral impairments that lead to neuronal death. A substantial number of studies encourage targeting the skewness in the production and degradation of amyloid-β could be among the promising therapies in the disease. Neuronal autophagy has emerged for an essential role in the degradation of such toxic aggregate-prone proteins in various neurodegenerative diseases. We profiled a small library of common dietary compounds and identified those that can enhance autophagy in neuronal cells. Here we noted naringenin in silico exhibits a robust affinity with AMP-activated protein kinase (AMPK) and upregulated AMPK-mediated autophagy signaling in neurons. Naringenin can induce autophagy promoting proteins such as ULK1, Beclin1, ATG5, and ATG7 in Neuro2a cells and primary mouse neurons as well. The knockdown of AMPK by siRNA-AMPK was complemented by naringenin that restored transcript levels of AMPK. Further, naringenin can reduce the levels of Aβ at a nontoxic concentration from neuronal cells. Moreover, it maintained the mitochondrial membrane potential and resisted reactive oxygen species production, which led to the protection against Aβ1–42 evoked neurotoxicity. This highlights the neuroprotective potential of naringenin that can be developed as an anti-amyloidogenic nutraceutical.







Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- AMPK:
-
AMP-activated protein kinase
- MAP 2:
-
Microtubule-associated protein
- GFAP :
-
Glial fibrillary acidic protein
- LC3B:
-
Microtubule-associated protein 1 light chain 3 beta
- mTOR :
-
Mammalian target of rapamycin
- Aβ :
-
Amyloid beta
- ULK1:
-
Unc-51-like kinase
- ATG :
-
Autophagy-related protein
- SQSTM1/P62:
-
Sequestosome 1 protein)
- NGN:
-
Naringenin
- Baf:
-
Bafilomycin A1
- TMRE:
-
Tetramethylrhodamine ethyl ester
- MTT :
-
Thiazolyl blue tetrazolium bromide
- LDH :
-
Lactate dehydrogenase
- AICAR:
-
5-Aminoimidazole-4-carboxamide ribonucleotide
- MMP:
-
Mitochondrial membrane potential
- ROS :
-
Reactive oxygen species
- DCFH2-DA:
-
2', 7'-dichloro-dihydro fluorescein diacetate
References
Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.
Leong YQ, Ng KY, Chye SM et al (2020) Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metab Brain Dis 35:11–30
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer's disease. Cold Spring Harb Perspect Med 1(1):a006189
Panza F, Lozupone M, Logroscino G, Imbimbo BP (2019) A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol 15(2):73–88
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R et al (2014) Alzheimer’s Disease Cooperative Study Steering Committee, Solanezumab Study Group Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 23(370(4)):311–321
Salloway S, Sperling R, Fox NC (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370(4):322–333
Anguiano J, Garner TP, Mahalingam M, Das BC, Gavathiotis E, Cuervo AM (2013) Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol 9:374–382
Anding AL, Baehrecke EH (2017) Cleaning house: selective autophagy of organelles. Dev Cell 41(1):10–22
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu H, Nixon RA (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci 28(27):6926–6937
Chen Y, Xu S, Wang N, Peng P, Yu Y, Zhang L, Ying Z, Wang H (2019) Dynasore suppresses mTORC1 activity and induces autophagy to regulate the clearance of protein aggregates in neurodegenerative diseases. Neurotox Res 36:108–116
Ntsapi C, Lumkwana D, Swart C, du Toit A, Loos B (2018) New insights into autophagy dysfunction related to amyloid beta toxicity and neuropathology in Alzheimer’s disease. Int Rev Cell Mol Biol 336:321–361
Vingtdeux V, Chandakkar P, Zhao H, d'Abramo C, Davies P, Marambaud P (2011) Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. FASEB J 25:219–231
Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M (2011) AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem 118:460–474
Park SY, Lee HR, Lee WS, Shin HK, Kim HY, Hong KW (2016) Cilostazol modulates autophagic degradation of beta-amyloid peptide via SIRT1-coupled LKB1/AMPK alpha signaling in neuronal cells. PLoS One 11:e0160620
Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z (2011) Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neuro-Signals. 19:163–174
Walter C, Clemens LE, Muller AJ, Fallier-Becker P, Proikas-Cezanne T, Riess O (2016) Activation of AMPK-induced autophagy ameliorates Huntington disease pathology in vitro. Neuropharmacology. 108:24–38
Heras-Sandoval D, Perez-Rojas JM, Pedraza-Chaverri J (2020) Novel compounds for the modulation of mTOR and autophagy to treat neurodegenerative diseases. Cell Signal 65:109442
Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J et al (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-b peptide metabolism. J Biol Chem 285:9100–9113
Zobeiri M, Belwal T, Parvizi F, Naseri R, Farzaei MH, Nabavi SF, Sureda A, Nabavi SM (2018) Naringenin and its nano-formulations for fatty liver: Cellular modes of action and clinical perspective. Curr Pharm Biotechnol 19:196–205
Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, Sharifi-Rad J (2019) The therapeutic potential of Naringenin: a review of clinical trials. Pharmaceuticals (Basel) 12(1):E11
Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ (2004) Flavonoid permeability across an in-situ model of the blood–brain barrier. Free Radic Biol Med 36:592–604
Wang Q, Yang J, Zhang XM, Zhou L, Liao XL, Yang B (2015) Practical synthesis of naringenin. J Chem Res 39:455–457
Wang GQ, Zhang B, He XM, Li DD, Shi JS, Zhang F (2019) Naringenin targets on astroglial Nrf2 to support dopaminergic neurons. Pharmacol Res 139:452–459
Hegazy HG, Ali EHA, Sabry HA (2016) The neuroprotective action of naringenin on oseltamivir (Tamiflu) treated male rats. J Basic Appl Zool 77:83–90
Khan MB, Khan MM, Khan A, Ahmed ME, Ishrat T, Tabassum R, Vaibhav K, Islam F (2012) Naringenin ameliorates Alzheimer’s disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular-streptozotocin in rat model. Neurochem Int 61(7):1081–1093
Chen C, Wei YZ, He XM, Li DD, Wang GQ, Li JJ, Zhang F (2019) Naringenin produces neuroprotection against LPS-induced dopamine neurotoxicity via the inhibition of microglial NLRP3 inflammasome activation. Front Immunol 10:936
Yang Z, Kuboyama T, Tohda C (2019) Naringenin promotes microglial M2 polarization and Aβ degradation enzyme expression. Phytother Res 33(4):1114–1121
Siddiqi FH, Menzies FM, Lopez A et al (2019) Felodipine induces autophagy in mouse brains with pharmacokinetics amenable to repurposing. Nat Commun 10:1817
Dar NJ, Satti NK, Dutt P et al (2018) Attenuation of glutamate-induced Excitotoxicity by Withanolide-a in neuron-like cells: Role for PI3K/Akt/MAPK signaling pathway. Mol Neurobiol 55:2725–2739
Gupta M, Wani A, Ahsan AU, Chopra M, Vishwakarma RA, Singh G, Kumar A (2018) Soluble Aβ1-42 suppresses TNF-α and activates NLRP3 inflammasome in THP-1 macrophages. Cytokine. 111:84–87
Klionsky DJ, Abdelmohsen K, Abe A et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 12(1):1–222
Gupta S, Ahsan AU, Wani A, Khajuria V, Nazir LA, Sharma S, Bhagat A, Sharma PR et al (2018) The amino analogue of β-boswellic acid efficiently attenuates the release of pro-inflammatory mediators than its parent compound through the suppression of NF-κB/IκBα signalling axis. Cytokine. 107:93–104
Wani A, Gupta M, Ahmad M, Shah AM, Ahsan AU, Qazi PH, Malik F, Singh G et al (2019) Alborixin clears amyloid-β by inducing autophagy through PTEN-mediated inhibition of the AKT pathway. Autophagy. 15(10):1810–1828
Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA (2011) Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques. 50(2):98–115
Kaushal S, Ahsan AU, Sharma VL, Chopra M (2019) Epigallocatechin gallate attenuates arsenic induced genotoxicity via regulation of oxidative stress in balb/C mice. Mol Biol Rep 46:5355–5369
Tanida I, Ueno T, Kominami E (2008) LC3 and Autophagy. Methods in Molecular Biology (Clifton, N.J.). 445:77-88.
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141
Tan CC, Yu JT, Tan MS, Jiang T, Zhu XC, Tan L (2014) Autophagy in aging and neurodegenerative diseases: Implications for pathogenesis and therapy. Neurobiol Aging 35:941–957
Cai Z, Zhao B, Li K, Zhang L, Li C, Quazi SH, Tan Y (2012) Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer’s disease? J Neurosci Res 90(6):1105–1118
Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19:983–997
Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G (2017) Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov 16(7):487–511
Maher P, Dargusch R, Ehren JL, Okada S, Sharma K, Schubert D (2011) Fisetin lowers methylglyoxal dependent protein glycation and limits the complications of diabetes. PLoS One 6:e21226
Saenz J, Santa-María C, Reyes-Quiroz ME et al (2018) Grapefruit flavonoid Naringenin regulates the expression of LXRα in THP-1 macrophages by modulating AMP-activated protein kinase. Mol Pharm 15(5):1735–1745
Li S, Zhang Y, Sun Y et al (2019) Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr Diabetes 9:28
Wu L, Lin C, Lin H et al (2016) Naringenin suppresses neuroinflammatory responses through inducing suppressor of cytokine signaling 3 expression. Mol Neurobiol 53:1080–1091
Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB (2013) Autophagy: regulation and role in development. Autophagy. 9(7):951–972
Nikoletopoulou V, Tavernarakis N (2018) Regulation and roles of autophagy at synapses. Trends Cell Biol 28:8
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B et al (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates β-amyloid accumulation in mice. J Clin Invest 118(6):2190–2199
Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A et al (2017) Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 93:1015–1034
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F (2018) Oxidative stress and the β-amyloid peptide in Alzheimer’s disease. Redox Biol 14:450–464
Kapogiannis D, Mattson MP (2011) Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol 10:187–198
Lou H, Jing X, Wei X, Shi H, Ren D, Zhang X (2014) Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology. 79:380–388
Acknowledgments
We are thankful to UGC (University Grants Commission) for providing financial assistance to Aitizaz Ul Ahsan to carry out this research. We are immensely grateful to Dr. Ajay Kumar for providing his lab during experimental work. We thank Dr. Akeel for his kind help in gifting plasmids. We cannot forget to mention Dr. Abid Hamid for his support and critical suggestions. Thanks are due to Dr. P.R. Sharma for assisting in microscopy and lastly to IIIM, Jammu Kashmir, for keeping facilities available. Dr. Mehak Gupta, Manisha, Mir Faheem Mohammad, Masroor Ahmad, and Adil Qadir, thanks for the help.
Author information
Authors and Affiliations
Contributions
A.U.A planned and performed the experiments and analyzed the data and compiled the manuscript. A.W. helped in designing some experiments. V.L.S and M.C. revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
All animal procedures were approved by the Institutional Animals Ethics Committee IAEC (PU/IAEC/S/15/86), Panjab University Chandigarh.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Naringenin confers neuroprotection against soluble Aβ-evoked neurotoxicity in neuronal cells.
• AMPK-mediated autophagy by naringenin mediates its neuroprotection.
• This study not only establishes the underlying mechanism but also provides the basis for finding the strategy in treating the Aβ pathology.
Electronic Supplementary Material

Supplementary Fig. 1
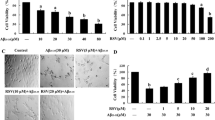
(a) Screening of various compounds for AMPK induction with metformin (Met) as the standard. (b) Cell viability assay of naringenin at different concentrations in differentiated N2a cells and primary mouse neurons. (c) In-silco evaluation for molecular docking of naringenin with AMPK compared to AICAR. (d) Induction of autophagy by naringenin at different concentrations in N2a cells(PNG 851 kb)
Rights and permissions
About this article
Cite this article
Ahsan, A.U., Sharma, V.L., Wani, A. et al. Naringenin Upregulates AMPK-Mediated Autophagy to Rescue Neuronal Cells From β-Amyloid (1–42) Evoked Neurotoxicity. Mol Neurobiol 57, 3589–3602 (2020). https://doi.org/10.1007/s12035-020-01969-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01969-4