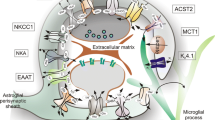
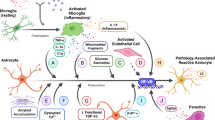
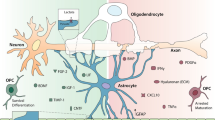
Abstract
Transforming growth factor betas (TGF-βs) are known as multifunctional growth factors that participate in the regulation of key events of development, disease, and tissue repair. In the brain, TGF-β1 has been widely recognized as an injury-related cytokine, particularly associated with astrocyte scar formation in response to brain injury. In the last decade, however, evidence has indicated that in addition to its role in brain injury, TGF-β1 might be a crucial regulator of cell survival and differentiation, brain homeostasis, angiogenesis, memory formation, and neuronal plasticity. In this review, we will discuss the emerging scenario of TGF-β1 as a key regulator of astrocyte differentiation and function and the implications of TGF-β1 as a novel mediator of cellular interactions in the central nervous system. First, we will discuss the cellular and molecular basis underlying the effect of TGF-β on astrocyte generation and its impact on angiogenesis and blood-brain barrier function. Then, we will focus on the role of astrocytes in the development and remodeling of synapses and the role of TGF-β1 as a new mediator of these events. Furthermore, we present seminal data that contributed to the emerging concept that astrocyte dysfunction might be associated with neurodegenerative diseases, with a special focus on Alzheimer’s disease, and discuss the pros and cons of TGF-β signaling deficits in these processes. Finally, we argue that understanding how astrocytic signals, such as TGF-β1, regulate brain function might offer new insights into human learning, memory, and cognition, and ultimately, this understanding may provide new targets for the treatment of neurological diseases.




Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- AβO:
-
Aβ oligomers
- BBB:
-
Blood-brain barrier
- BDNF:
-
Brain-derived neurotrophic factor
- BLBP:
-
Brain lipid binding protein
- BMP:
-
Bone morphogenetic protein
- CaMKII:
-
Ca2+/calmodulin-dependent protein kinase II
- CNS:
-
Central nervous system
- CR3:
-
C3 receptor
- CREB:
-
AMP responsive element binding transcription factor
- ECM:
-
Extracellular matrix
- ECs:
-
Endothelial cells
- FGFb:
-
Fibroblast growth factor beta
- GDFs:
-
Growth and differentiation factors
- GFAP:
-
Glial fibrillary acidic protein
- GGT:
-
γ-Glutamyl transferase
- GLAST:
-
Astrocyte-specific glutamate-aspartate transporter
- Gpr124:
-
G protein-coupled endothelial receptor 124
- HHT2:
-
Hereditary hemorrhagic telangiectasia type 2
- i.c.v.:
-
Intracerebroventricular injection
- IL-6:
-
Interleukin 6
- JNK:
-
c-jun-N-terminal kinase
- LAP:
-
Latency-associated peptide
- LIF:
-
Leukemia inhibitory factor
- LPS:
-
Lipopolysaccharide
- LTD:
-
Long-term depression
- LTP:
-
Long-term potentiation
- MAPK:
-
Mitogen-activated protein kinase
- NPCs:
-
Neural progenitor cells
- PAI-1:
-
Plasminogen activator inhibitor-1
- PAP:
-
Perisynaptic astrocyte processes
- PD:
-
Parkinson’s disease
- PDGF-b:
-
Platelet-derived growth factor beta
- PI3K:
-
Phosphatidylinositol-3 kinase
- PNVP:
-
Perivascular neural plexus
- PNS:
-
Peripheral nervous system
- RG:
-
Radial glia cells
- RGCs:
-
Retinal ganglion cells
- SASP:
-
Senescence-associated secretory phenotype
- SVZ:
-
Subventricular zone
- TGF-β1:
-
Transforming growth factor beta one
- TβR1:
-
TGF-β type I receptor
- TβR2:
-
TGF-β type II receptor
- TβR3:
-
TGF-β type III receptor
- TNF-α:
-
Tumor necrosis factor alpha
- TSP:
-
Thrombospondin
- VEGF-A:
-
Vascular endothelial growth factor A
- VZ:
-
Ventricular zone
- ZO-1:
-
Zonula occludens-1
- β–Gal:
-
β-galactosidase
References
Verkhratsky A, Nedergaard M (2018) Physiology of Astroglia. Physiol Rev 98(1):239–389. https://doi.org/10.1152/physrev.00042.2016
Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B (2008) Neocortical glial cell numbers in human brains. Neurobiol Aging 29(11):1754–1762. https://doi.org/10.1016/j.neurobiolaging.2007.04.013
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26(10):523–530. https://doi.org/10.1016/j.tins.2003.08.008
Sun W, Cornwell A, Li J, Peng S, Osorio MJ, Aalling N, Wang S, Benraiss A et al (2017) SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci 37(17):4493–4507. https://doi.org/10.1523/JNEUROSCI.3199-16.2017
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A 115(8):E1896–E1905. https://doi.org/10.1073/pnas.1800165115
Kettenmann H, Verkhratsky A (2008) Neuroglia: the 150 years after. Trends Neurosci 31(12):653–659. https://doi.org/10.1016/j.tins.2008.09.003
Feig SL, Haberly LB (2011) Surface-associated astrocytes, not endfeet, form the glia limitans in posterior piriform cortex and have a spatially distributed, not a domain, organization. J Comp Neurol 519(10):1952–1969. https://doi.org/10.1002/cne.22615
Chan-Palay V, Palay SL (1972) The form of velate astrocytes in the cerebellar cortex of monkey and rat: high voltage electron microscopy of rapid Golgi preparations. Z Anat Entwicklungsgesch 138(1):1–19
Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45. https://doi.org/10.1007/978-1-61779-452-0_3
Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD et al (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29(10):3276–3287. https://doi.org/10.1523/JNEUROSCI.4707-08.2009
Oberheim NA, Wang X, Goldman S, Nedergaard M (2006) Astrocytic complexity distinguishes the human brain. Trends Neurosci 29(10):547–553. https://doi.org/10.1016/j.tins.2006.08.004
Lavialle M, Aumann G, Anlauf E, Prols F, Arpin M, Derouiche A (2011) Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A 108(31):12915–12919. https://doi.org/10.1073/pnas.1100957108
Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Invest 122(4):1164–1171. https://doi.org/10.1172/JCI58644
Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275(Pt 3):305–315. https://doi.org/10.1016/j.expneurol.2015.03.020
Blondel O, Collin C, McCarran WJ, Zhu S, Zamostiano R, Gozes I, Brenneman DE, McKay RD (2000) A glia-derived signal regulating neuronal differentiation. J Neurosci 20(21):8012–8020
Goritz C, Mauch DH, Pfrieger FW (2005) Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci 29(2):190–201. https://doi.org/10.1016/j.mcn.2005.02.006
Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science 294(5545):1354–1357. https://doi.org/10.1126/science.294.5545.1354
Hu R, Cai WQ, Wu XG, Yang Z (2007) Astrocyte-derived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons. Neuroscience 144(4):1229–1240. https://doi.org/10.1016/j.neuroscience.2006.09.056
Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC (2002) Control of synaptic strength by glial TNFalpha. Science 295(5563):2282–2285. https://doi.org/10.1126/science.1067859
Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF et al (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120(3):421–433. https://doi.org/10.1016/j.cell.2004.12.020
Parpura V, Haydon PG (2000) Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A 97(15):8629–8634
Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K (2003) Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci U S A 100(19):11023–11028. https://doi.org/10.1073/pnas.1834448100
Schell MJ, Molliver ME, Snyder SH (1995) D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A 92(9):3948–3952
Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S (2003) Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A 100(25):15194–15199. https://doi.org/10.1073/pnas.2431073100
Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH (2006) Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125(4):775–784. https://doi.org/10.1016/j.cell.2006.02.051
Henneberger C, Papouin T, Oliet SH, Rusakov DA (2010) Long-term potentiation depends on release of D-serine from astrocytes. Nature 463(7278):232–236. https://doi.org/10.1038/nature08673
Pannasch U, Freche D, Dallerac G, Ghezali G, Escartin C, Ezan P, Cohen-Salmon M, Benchenane K et al (2014) Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci 17(4):549–558. https://doi.org/10.1038/nn.3662
Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18(7):942–952. https://doi.org/10.1038/nn.4043
Ben Haim L, Rowitch DH (2017) Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18(1):31–41. https://doi.org/10.1038/nrn.2016.159
Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS et al (2017) Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95(3):531–549 e539. https://doi.org/10.1016/j.neuron.2017.06.029
Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ (2018) The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep 22(1):269–285. https://doi.org/10.1016/j.celrep.2017.12.039
Stipursky J, Gomes FC (2007) TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 55(10):1023–1033. https://doi.org/10.1002/glia.20522
Spohr TC, Choi JW, Gardell SE, Herr DR, Rehen SK, Gomes FC, Chun J (2008) Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation. J Biol Chem 283(12):7470–7479. https://doi.org/10.1074/jbc.M707758200
Diniz LP, Pereira Matias IC, Garcia M, Alcantara Gomes FC (2014) Astrocytic control of neural circuit formation: Highlights on TGF-beta signaling. Neurochem Int 78:18–27. https://doi.org/10.1016/j.neuint.2014.07.008
Bialas AR, Stevens B (2013) TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16(12):1773–1782. https://doi.org/10.1038/nn.3560
Sultan S, Li L, Moss J, Petrelli F, Casse F, Gebara E, Lopatar J, Pfrieger FW et al (2015) Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88(5):957–972. https://doi.org/10.1016/j.neuron.2015.10.037
Chung WS, Allen NJ, Eroglu C (2015) Astrocytes control synapse formation, function, and elimination. Cold Spring Harb Perspect Biol 7(9):a020370. https://doi.org/10.1101/cshperspect.a020370
Diniz LP, Tortelli V, Matias I, Morgado J, Bergamo Araujo AP, Melo HM, Seixas da Silva GS, Alves-Leon SV et al (2017) Astrocyte transforming growth factor beta 1 protects synapses against Abeta oligomers in Alzheimer’s disease model. J Neurosci 37(28):6797–6809. https://doi.org/10.1523/JNEUROSCI.3351-16.2017
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46(6):957–967. https://doi.org/10.1016/j.immuni.2017.06.006
Moraes CA, Santos G, de Sampaio e Spohr TC, D’Avila JC, Lima FR, Benjamim CF, Bozza FA, Gomes FC (2015) Activated microglia-induced deficits in excitatory synapses through IL-1beta: implications for cognitive impairment in sepsis. Mol Neurobiol 52(1):653–663. https://doi.org/10.1007/s12035-014-8868-5
Weiss A, Attisano L (2013) The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol 2(1):47–63. https://doi.org/10.1002/wdev.86
de Caestecker M (2004) The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev 15(1):1–11
Gantus MA, Alves LM, Stipursky J, Souza EC, Teodoro AJ, Alves TR, Carvalho DP, Martinez AM et al (2011) Estradiol modulates TGF-beta1 expression and its signaling pathway in thyroid stromal cells. Mol Cell Endocrinol 337(1–2):71–79. https://doi.org/10.1016/j.mce.2011.02.001
Massague J, Gomis RR (2006) The logic of TGFbeta signaling. FEBS Lett 580(12):2811–2820. https://doi.org/10.1016/j.febslet.2006.04.033
Tinoco-Veras CM, Santos A, Stipursky J, Meloni M, Araujo APB, Foschetti DA, Lopez-Urena D, Quesada-Gomez C, Leitao RFC, Gomes FCA, Brito GAC (2017) Transforming growth factor beta1/SMAD signaling pathway activation protects the intestinal epithelium from Clostridium difficile toxin A-induced damage. Infect Immun 85(10). https://doi.org/10.1128/IAI.00430-17
Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB (2015) Latent TGF-beta-binding proteins. Matrix Biol 47:44–53. https://doi.org/10.1016/j.matbio.2015.05.005
Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB (1997) Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int 51(5):1376–1382
Guo X, Wang XF (2009) Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res 19(1):71–88. https://doi.org/10.1038/cr.2008.302
Miyazawa K, Miyazono K (2017) Regulation of TGF-beta family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol 9(3). https://doi.org/10.1101/cshperspect.a022095
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113(6):685–700
Romao LF, Sousa Vde O, Neto VM, Gomes FC (2008) Glutamate activates GFAP gene promoter from cultured astrocytes through TGF-beta1 pathways. J Neurochem 106(2):746–756. https://doi.org/10.1111/j.1471-4159.2008.05428.x
Stipursky J, Francis D, Gomes FC (2012) Activation of MAPK/PI3K/SMAD pathways by TGF-beta(1) controls differentiation of radial glia into astrocytes in vitro. Dev Neurosci 34(1):68–81. https://doi.org/10.1159/000338108
Javelaud D, Mauviel A (2005) Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene 24(37):5742–5750. https://doi.org/10.1038/sj.onc.1208928
O'Brien CE, Bonanno L, Zhang H, Wyss-Coray T (2015) Beclin 1 regulates neuronal transforming growth factor-beta signaling by mediating recycling of the type I receptor ALK5. Mol Neurodegener 10:69. https://doi.org/10.1186/s13024-015-0065-0
Brionne TC, Tesseur I, Masliah E, Wyss-Coray T (2003) Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40(6):1133–1145
Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C et al (2008) Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci 28(2):434–446. https://doi.org/10.1523/JNEUROSCI.4374-07.2008
Ageta H, Murayama A, Migishima R, Kida S, Tsuchida K, Yokoyama M, Inokuchi K (2008) Activin in the brain modulates anxiety-related behavior and adult neurogenesis. PLoS One 3(4):e1869. https://doi.org/10.1371/journal.pone.0001869
Miller MW (2003) Expression of transforming growth factor-beta in developing rat cerebral cortex: effects of prenatal exposure to ethanol. J Comp Neurol 460(3):410–424. https://doi.org/10.1002/cne.10658
Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF (2005) Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25(44):10074–10086. https://doi.org/10.1523/JNEUROSCI.3114-05.2005
Diniz LP, Tortelli V, Garcia MN, Araujo AP, Melo HM, Seixas da Silva GS, De Felice FG, Alves-Leon SV, de Souza JM, Romao LF, Castro NG, Gomes FC (2014) Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia. https://doi.org/10.1002/glia.22713
Diniz LP, Almeida JC, Tortelli V, Vargas Lopes C, Setti-Perdigao P, Stipursky J, Kahn SA, Romao LF et al (2012) Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J Biol Chem 287(49):41432–41445. https://doi.org/10.1074/jbc.M112.380824
Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD (2010) TGF-beta signaling specifies axons during brain development. Cell 142(1):144–157. https://doi.org/10.1016/j.cell.2010.06.010
Stipursky J, Francis D, Dezonne RS, Bergamo de Araujo AP, Souza L, Moraes CA, Alcantara Gomes FC (2014) TGF-beta1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo. Front Cell Neurosci 8:393. https://doi.org/10.3389/fncel.2014.00393
Sousa Vde O, Romao L, Neto VM, Gomes FC (2004) Glial fibrillary acidic protein gene promoter is differently modulated by transforming growth factor-beta 1 in astrocytes from distinct brain regions. Eur J Neurosci 19(7):1721–1730. https://doi.org/10.1111/j.1460-9568.2004.03249.x
Hellbach N, Weise SC, Vezzali R, Wahane SD, Heidrich S, Roidl D, Pruszak J, Esser JS et al (2014) Neural deletion of Tgfbr2 impairs angiogenesis through an altered secretome. Hum Mol Genet 23(23):6177–6190. https://doi.org/10.1093/hmg/ddu338
Hirota S, Clements TP, Tang LK, Morales JE, Lee HS, Oh SP, Rivera GM, Wagner DS et al (2015) Neuropilin 1 balances beta8 integrin-activated TGFbeta signaling to control sprouting angiogenesis in the brain. Development 142(24):4363–4373. https://doi.org/10.1242/dev.113746
Siqueira M, Francis D, Gisbert D, Gomes FCA, Stipursky J (2017) Radial glia cells control angiogenesis in the developing cerebral cortex through TGF-beta1 signaling. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0557-8
Mecha M, Rabadan MA, Pena-Melian A, Valencia M, Mondejar T, Blanco MJ (2008) Expression of TGF-betas in the embryonic nervous system: analysis of interbalance between isoforms. Dev Dyn 237(6):1709–1717. https://doi.org/10.1002/dvdy.21558
Galter D, Bottner M, Unsicker K (1999) Developmental regulation of the serotonergic transmitter phenotype in rostral and caudal raphe neurons by transforming growth factor-betas. J Neurosci Res 56(5):531–538. https://doi.org/10.1002/(SICI)1097-4547(19990601)56:5<531::AID-JNR8>3.0.CO;2-O
Böttner M, Krieglstein K, Unsicker K (2000) The transforming growth factor-βs: structure, signaling, and roles in nervous system development and functions. J Neurochem 75(6):2227–2240
Bottner M, Unsicker K, Suter-Crazzolara C (1996) Expression of TGF-beta type II receptor mRNA in the CNS. Neuroreport 7(18):2903–2907
Tomoda T, Shirasawa T, Yahagi YI, Ishii K, Takagi H, Furiya Y, Arai KI, Mori H et al (1996) Transforming growth factor-beta is a survival factor for neonate cortical neurons: coincident expression of type I receptors in developing cerebral cortices. Dev Biol 179(1):79–90. https://doi.org/10.1006/dbio.1996.0242
Vivien D, Bernaudin M, Buisson A, Divoux D, MacKenzie ET, Nouvelot A (1998) Evidence of type I and type II transforming growth factor-beta receptors in central nervous tissues: changes induced by focal cerebral ischemia. J Neurochem 70(6):2296–2304
de Sampaio e Spohr TC, Martinez R, da Silva EF, Neto VM, Gomes FC (2002) Neuro-glia interaction effects on GFAP gene: a novel role for transforming growth factor-beta1. Eur J Neurosci 16(11):2059–2069
Goumans MJ, Mummery C (2000) Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44(3):253–265
Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ (1995) Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 121(6):1845–1854
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C et al (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359(6397):693–699. https://doi.org/10.1038/359693a0
Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB et al (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90(2):770–774
Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K et al (2000) Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A 97(6):2626–2631
Oshima M, Oshima H, Taketo MM (1996) TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol 179(1):297–302. https://doi.org/10.1006/dbio.1996.0259
Arnold TD, Niaudet C, Pang MF, Siegenthaler J, Gaengel K, Jung B, Ferrero GM, Mukouyama YS et al (2014) Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development 141(23):4489–4499. https://doi.org/10.1242/dev.107193
Koeglsperger T, Li S, Brenneis C, Saulnier JL, Mayo L, Carrier Y, Selkoe DJ, Weiner HL (2013) Impaired glutamate recycling and GluN2B-mediated neuronal calcium overload in mice lacking TGF-beta1 in the CNS. Glia 61(6):985–1002. https://doi.org/10.1002/glia.22490
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184. https://doi.org/10.1146/annurev.neuro.051508.135600
Stipursky J, Romao L, Tortelli V, Neto VM, Gomes FC (2011) Neuron-glia signaling: implications for astrocyte differentiation and synapse formation. Life Sci 89(15–16):524–531. https://doi.org/10.1016/j.lfs.2011.04.005
Hebert JM, Fishell G (2008) The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci 9(9):678–685. https://doi.org/10.1038/nrn2463
Toresson H, Potter SS, Campbell K (2000) Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development 127(20):4361–4371
Stoykova A, Treichel D, Hallonet M, Gruss P (2000) Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci 20(21):8042–8050
Kriegstein AR, Gotz M (2003) Radial glia diversity: a matter of cell fate. Glia 43(1):37–43. https://doi.org/10.1002/glia.10250
Pinto L, Gotz M (2007) Radial glial cell heterogeneity—the source of diverse progeny in the CNS. Prog Neurobiol 83(1):2–23. https://doi.org/10.1016/j.pneurobio.2007.02.010
deAzevedo LC, Fallet C, Moura-Neto V, Daumas-Duport C, Hedin-Pereira C, Lent R (2003) Cortical radial glial cells in human fetuses: depth-correlated transformation into astrocytes. J Neurobiol 55(3):288–298. https://doi.org/10.1002/neu.10205
Freeman MR, Rowitch DH (2013) Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80(3):613–623. https://doi.org/10.1016/j.neuron.2013.10.034
Schmechel DE, Rakic P (1979) A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 156(2):115–152
Takahashi T, Misson JP, Caviness VS Jr (1990) Glial process elongation and branching in the developing murine neocortex: a qualitative and quantitative immunohistochemical analysis. J Comp Neurol 302(1):15–28. https://doi.org/10.1002/cne.903020103
Voigt T (1989) Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol 289(1):74–88. https://doi.org/10.1002/cne.902890106
Masjosthusmann S, Becker D, Petzuch B, Klose J, Siebert C, Deenen R, Barenys M, Baumann J et al (2018) A transcriptome comparison of time-matched developing human, mouse and rat neural progenitor cells reveals human uniqueness. Toxicol Appl Pharmacol 354:40–55. https://doi.org/10.1016/j.taap.2018.05.009
Barnabe-Heider F, Wasylnka JA, Fernandes KJ, Porsche C, Sendtner M, Kaplan DR, Miller FD (2005) Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48(2):253–265. https://doi.org/10.1016/j.neuron.2005.08.037
Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T (1999) Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284(5413):479–482
Gomes FC, Garcia-Abreu J, Galou M, Paulin D, Moura Neto V (1999) Neurons induce GFAP gene promoter of cultured astrocytes from transgenic mice. Glia 26(2):97–108
Garcia-Marques J, Lopez-Mascaraque L (2013) Clonal identity determines astrocyte cortical heterogeneity. Cereb Cortex 23(6):1463–1472. https://doi.org/10.1093/cercor/bhs134
Grove EA, Williams BP, Li DQ, Hajihosseini M, Friedrich A, Price J (1993) Multiple restricted lineages in the embryonic rat cerebral cortex. Development 117(2):553–561
Luskin MB, McDermott K (1994) Divergent lineages for oligodendrocytes and astrocytes originating in the neonatal forebrain subventricular zone. Glia 11(3):211–226. https://doi.org/10.1002/glia.440110302
Parnavelas JG (1999) Glial cell lineages in the rat cerebral cortex. Exp Neurol 156(2):418–429. https://doi.org/10.1006/exnr.1999.7044
Buosi AS, Matias I, Araujo AP, Batista C, Gomes FC (2017) Heterogeneity in synaptogenic profile of astrocytes from different brain regions. Mol Neurobiol 55:751–762. https://doi.org/10.1007/s12035-016-0343-z
Rowitch DH, Kriegstein AR (2010) Developmental genetics of vertebrate glial-cell specification. Nature 468(7321):214–222. https://doi.org/10.1038/nature09611
Kim JH, Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW (2006) Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol 39(4):339–345
Liebner S, Czupalla CJ, Wolburg H (2011) Current concepts of blood-brain barrier development. Int J Dev Biol 55(4–5):467–476. https://doi.org/10.1387/ijdb.103224sl
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12):1584–1596. https://doi.org/10.1038/nm.3407
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7(1):41–53. https://doi.org/10.1038/nrn1824
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3(6):401–410. https://doi.org/10.1038/nrc1093
Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C (2004) Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn 231(3):503–509. https://doi.org/10.1002/dvdy.20148
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C et al (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161(6):1163–1177. https://doi.org/10.1083/jcb.200302047
James JM, Gewolb C, Bautch VL (2009) Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development 136(5):833–841. https://doi.org/10.1242/dev.028845
Pepper MS (1997) Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 8(1):21–43
Ferrari G, Cook BD, Terushkin V, Pintucci G, Mignatti P (2009) Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol 219(2):449–458. https://doi.org/10.1002/jcp.21706
Nguyen HL, Lee YJ, Shin J, Lee E, Park SO, McCarty JH, Oh SP (2011) TGF-beta signaling in endothelial cells, but not neuroepithelial cells, is essential for cerebral vascular development. Lab Investig 91(11):1554–1563. https://doi.org/10.1038/labinvest.2011.124
Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P et al (2001) Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J 20(7):1663–1673. https://doi.org/10.1093/emboj/20.7.1663
Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P et al (2011) Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc Natl Acad Sci U S A 108(7):2807–2812. https://doi.org/10.1073/pnas.1019761108
Nakagawa T, Li JH, Garcia G, Mu W, Piek E, Bottinger EP, Chen Y, Zhu HJ et al (2004) TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int 66(2):605–613. https://doi.org/10.1111/j.1523-1755.2004.00780.x
Goumans MJ, Lebrin F, Valdimarsdottir G (2003) Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med 13(7):301–307
Stefansson S, Lawrence DA (1996) The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature 383(6599):441–443. https://doi.org/10.1038/383441a0
Stefansson S, Petitclerc E, Wong MK, McMahon GA, Brooks PC, Lawrence DA (2001) Inhibition of angiogenesis in vivo by plasminogen activator inhibitor-1. J Biol Chem 276(11):8135–8141. https://doi.org/10.1074/jbc.M007609200
Wu J, Strawn TL, Luo M, Wang L, Li R, Ren M, Xia J, Zhang Z et al (2015) Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-alphaVbeta3 integrin cross talk. Arterioscler Thromb Vasc Biol 35(1):111–120. https://doi.org/10.1161/ATVBAHA.114.304554
Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P (2002) Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21(7):1743–1753. https://doi.org/10.1093/emboj/21.7.1743
David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S (2007) Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109(5):1953–1961. https://doi.org/10.1182/blood-2006-07-034124
Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P (2007) BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci 120(Pt 6):964–972. https://doi.org/10.1242/jcs.002949
Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M et al (1996) Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13(2):189–195. https://doi.org/10.1038/ng0696-189
Goumans MJ, Liu Z, ten Dijke P (2009) TGF-beta signaling in vascular biology and dysfunction. Cell Res 19(1):116–127. https://doi.org/10.1038/cr.2008.326
Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM et al (2004) Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J 23(20):4018–4028. https://doi.org/10.1038/sj.emboj.7600386
Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M (1992) Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem 267(27):19027–19030
McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J et al (2005) Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 132(1):165–176. https://doi.org/10.1242/dev.01551
Proctor JM, Zang K, Wang D, Wang R, Reichardt LF (2005) Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci 25(43):9940–9948. https://doi.org/10.1523/JNEUROSCI.3467-05.2005
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37(1):13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Merwin JR, Anderson JM, Kocher O, Van Itallie CM, Madri JA (1990) Transforming growth factor beta 1 modulates extracellular matrix organization and cell-cell junctional complex formation during in vitro angiogenesis. J Cell Physiol 142(1):117–128. https://doi.org/10.1002/jcp.1041420115
Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y (2004) Transforming growth factor-beta1 upregulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol 24(3):491–497
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468(7323):562–566. https://doi.org/10.1038/nature09513
Winkler EA, Bell RD, Zlokovic BV (2011) Central nervous system pericytes in health and disease. Nat Neurosci 14(11):1398–1405. https://doi.org/10.1038/nn.2946
Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM et al (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508(7494):55–60. https://doi.org/10.1038/nature13165
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J et al (2010) Pericytes regulate the blood-brain barrier. Nature 468(7323):557–561. https://doi.org/10.1038/nature09522
Lindahl P, Johansson BR, Leveen P, Betsholtz C (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277(5323):242–245
Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A et al (2011) Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 20(3):291–302. https://doi.org/10.1016/j.devcel.2011.01.011
Van Geest RJ, Klaassen I, Vogels IM, Van Noorden CJ, Schlingemann RO (2010) Differential TGF-{beta} signaling in retinal vascular cells: a role in diabetic retinopathy? Invest Ophthalmol Vis Sci 51(4):1857–1865. https://doi.org/10.1167/iovs.09-4181
Garcia CM, Darland DC, Massingham LJ, D'Amore PA (2004) Endothelial cell-astrocyte interactions and TGF beta are required for induction of blood-neural barrier properties. Brain Res Dev Brain Res 152(1):25–38. https://doi.org/10.1016/j.devbrainres.2004.05.008
Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N et al (2005) Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol 166(6):1883–1894
Charles AC, Dirksen ER, Merrill JE, Sanderson MJ (1993) Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia 7(2):134–145. https://doi.org/10.1002/glia.440070203
Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247(4941):470–473
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22(5):208–215
Nedergaard M (1994) Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263(5154):1768–1771
Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369(6483):744–747. https://doi.org/10.1038/369744a0
Pfrieger FW, Barres BA (1997) Synaptic efficacy enhanced by glial cells in vitro. Science 277(5332):1684–1687
Ullian EM, Sapperstein SK, Christopherson KS, Barres BA (2001) Control of synapse number by glia. Science 291(5504):657–661. https://doi.org/10.1126/science.291.5504.657
Clarke LE, Barres BA (2013) Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci 14(5):311–321. https://doi.org/10.1038/nrn3484
Gomez-Casati ME, Murtie JC, Rio C, Stankovic K, Liberman MC, Corfas G (2010) Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc Natl Acad Sci U S A 107(39):17005–17010. https://doi.org/10.1073/pnas.1008938107
Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA (2012) Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486(7403):410–414. https://doi.org/10.1038/nature11059
Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G et al (2011) Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A 108(32):E440–E449. https://doi.org/10.1073/pnas.1104977108
Araujo AP, Diniz LP, Eller CM, de Matos BG, Martinez R, Gomes FC (2016) Effects of transforming growth factor beta 1 in cerebellar development: role in synapse formation. Front Cell Neurosci 10:104. https://doi.org/10.3389/fncel.2016.00104
Chin J, Angers A, Cleary LJ, Eskin A, Byrne JH (1999) TGF-beta1 in Aplysia: role in long-term changes in the excitability of sensory neurons and distribution of TbetaR-II-like immunoreactivity. Learn Mem 6(3):317–330
Chin J, Liu RY, Cleary LJ, Eskin A, Byrne JH (2006) TGF-beta1-induced long-term changes in neuronal excitability in aplysia sensory neurons depend on MAPK. J Neurophysiol 95(5):3286–3290. https://doi.org/10.1152/jn.00770.2005
Rawson JM, Lee M, Kennedy EL, Selleck SB (2003) Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor mad. J Neurobiol 55(2):134–150. https://doi.org/10.1002/neu.10189
Dudu V, Bittig T, Entchev E, Kicheva A, Julicher F, Gonzalez-Gaitan M (2006) Postsynaptic mad signaling at the Drosophila neuromuscular junction. Curr Biol 16(7):625–635. https://doi.org/10.1016/j.cub.2006.02.061
Lee SH, Kim YJ, Choi SY (2016) BMP signaling modulates the probability of neurotransmitter release and readily releasable pools in Drosophila neuromuscular junction synapses. Biochem Biophys Res Commun 479(3):440–446. https://doi.org/10.1016/j.bbrc.2016.09.072
Kim MJ, O'Connor MB (2014) Anterograde Activin signaling regulates postsynaptic membrane potential and GluRIIA/B abundance at the Drosophila neuromuscular junction. PLoS One 9(9):e107443. https://doi.org/10.1371/journal.pone.0107443
Sulkowski M, Kim YJ, Serpe M (2014) Postsynaptic glutamate receptors regulate local BMP signaling at the Drosophila neuromuscular junction. Development 141(2):436–447. https://doi.org/10.1242/dev.097758
Caraci F, Gulisano W, Guida CA, Impellizzeri AA, Drago F, Puzzo D, Palmeri A (2015) A key role for TGF-beta1 in hippocampal synaptic plasticity and memory. Sci Rep 5:11252. https://doi.org/10.1038/srep11252
Depino AM, Lucchina L, Pitossi F (2011) Early and adult hippocampal TGF-beta1 overexpression have opposite effects on behavior. Brain Behav Immun 25(8):1582–1591. https://doi.org/10.1016/j.bbi.2011.05.007
Mathieu P, Piantanida AP, Pitossi F (2010) Chronic expression of transforming growth factor-beta enhances adult neurogenesis. Neuroimmunomodulation 17(3):200–201. https://doi.org/10.1159/000258723
Graciarena M, Depino AM, Pitossi FJ (2010) Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFbeta1 downregulation. Brain Behav Immun 24(8):1301–1309. https://doi.org/10.1016/j.bbi.2010.06.005
Lacmann A, Hess D, Gohla G, Roussa E, Krieglstein K (2007) Activity-dependent release of transforming growth factor-beta in a neuronal network in vitro. Neuroscience 150(3):647–657. https://doi.org/10.1016/j.neuroscience.2007.09.046
Heupel K, Sargsyan V, Plomp JJ, Rickmann M, Varoqueaux F, Zhang W, Krieglstein K (2008) Loss of transforming growth factor-beta 2 leads to impairment of central synapse function. Neural Dev 3:25. https://doi.org/10.1186/1749-8104-3-25
Fukushima T, Liu RY, Byrne JH (2007) Transforming growth factor-beta2 modulates synaptic efficacy and plasticity and induces phosphorylation of CREB in hippocampal neurons. Hippocampus 17(1):5–9. https://doi.org/10.1002/hipo.20243
Fong SW, McLennan IS, McIntyre A, Reid J, Shennan KI, Bewick GS (2010) TGF-beta2 alters the characteristics of the neuromuscular junction by regulating presynaptic quantal size. Proc Natl Acad Sci U S A 107(30):13515–13519. https://doi.org/10.1073/pnas.1001695107
Feng Z, Ko CP (2008) Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-beta1. J Neurosci 28(39):9599–9609. https://doi.org/10.1523/JNEUROSCI.2589-08.2008
Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, Sweedler JV, Pollegioni L et al (2012) Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex 22(3):595–606. https://doi.org/10.1093/cercor/bhr130
Weissberg I, Wood L, Kamintsky L, Vazquez O, Milikovsky DZ, Alexander A, Oppenheim H, Ardizzone C et al (2015) Albumin induces excitatory synaptogenesis through astrocytic TGF-beta/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol Dis 78:115–125. https://doi.org/10.1016/j.nbd.2015.02.029
Yu CY, Gui W, He HY, Wang XS, Zuo J, Huang L, Zhou N, Wang K et al (2014) Neuronal and astroglial TGFbeta-Smad3 signaling pathways differentially regulate dendrite growth and synaptogenesis. NeuroMolecular Med 16(2):457–472. https://doi.org/10.1007/s12017-014-8293-y
Bae JJ, Xiang YY, Martinez-Canabal A, Frankland PW, Yang BB, Lu WY (2011) Increased transforming growth factor-beta1 modulates glutamate receptor expression in the hippocampus. Int J Physiol Pathophysiol Pharmacol 3(1):9–20
Cekanaviciute E, Dietrich HK, Axtell RC, Williams AM, Egusquiza R, Wai KM, Koshy AA, Buckwalter MS (2014) Astrocytic TGF-beta signaling limits inflammation and reduces neuronal damage during central nervous system toxoplasma infection. J Immunol 193(1):139–149. https://doi.org/10.4049/jimmunol.1303284
Cekanaviciute E, Fathali N, Doyle KP, Williams AM, Han J, Buckwalter MS (2014) Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia 62(8):1227–1240. https://doi.org/10.1002/glia.22675
Bonansco C, Fuenzalida M (2016) Plasticity of hippocampal excitatory-inhibitory balance: missing the synaptic control in the epileptic brain. Neural Plast 2016:8607038. https://doi.org/10.1155/2016/8607038
Zhou YX, Zhao M, Li D, Shimazu K, Sakata K, Deng CX, Lu B (2003) Cerebellar deficits and hyperactivity in mice lacking Smad4. J Biol Chem 278(43):42313–42320. https://doi.org/10.1074/jbc.M308287200
Sun M, Gewirtz JC, Bofenkamp L, Wickham RJ, Ge H, O'Connor MB (2010) Canonical TGF-beta signaling is required for the balance of excitatory/inhibitory transmission within the hippocampus and prepulse inhibition of acoustic startle. J Neurosci 30(17):6025–6035. https://doi.org/10.1523/JNEUROSCI.0789-10.2010
Luo SX, Timbang L, Kim JI, Shang Y, Sandoval K, Tang AA, Whistler JL, Ding JB et al (2016) TGF-beta signaling in dopaminergic neurons regulates dendritic growth, excitatory-inhibitory synaptic balance, and reversal learning. Cell Rep 17(12):3233–3245. https://doi.org/10.1016/j.celrep.2016.11.068
Lochman I, Svachova V, Milkova Pavlikova K, Medricka H, Novak V, Trilecova L, Pavliska L, Prochazka V (2018) Serum cytokine and growth factor levels in children with autism spectrum disorder. Med Sci Monit 24:2639–2646. https://doi.org/10.12659/MSM.906817
Heinemann U, Kaufer D, Friedman A (2012) Blood-brain barrier dysfunction, TGFbeta signaling, and astrocyte dysfunction in epilepsy. Glia 60(8):1251–1257. https://doi.org/10.1002/glia.22311
Bahramabadi R, Fathollahi MS, Hashemi SM, Arababadi AS, Arababadi MS, Yousefi-Daredor H, Bidaki R, Khaleghinia M et al (2017) Serum levels of IL-6, IL-8, TNF-alpha, and TGF-beta in chronic HBV-infected patients: effect of depression and anxiety. Lab Med 49(1):41–46. https://doi.org/10.1093/labmed/lmx064
Cattaneo A, Cattane N, Malpighi C, Czamara D, Suarez A, Mariani N, Kajantie E, Luoni A et al (2018) FoxO1, A2M, and TGF-beta1: three novel genes predicting depression in gene X environment interactions are identified using cross-species and cross-tissues transcriptomic and miRNomic analyses. Mol Psychiatry. https://doi.org/10.1038/s41380-017-0002-4
Knoferle J, Ramljak S, Koch JC, Tonges L, Asif AR, Michel U, Wouters FS, Heermann S et al (2010) TGF-beta 1 enhances neurite outgrowth via regulation of proteasome function and EFABP. Neurobiol Dis 38(3):395–404. https://doi.org/10.1016/j.nbd.2010.02.011
Nakashima H, Tsujimura K, Irie K, Ishizu M, Pan M, Kameda T, Nakashima K (2018) Canonical TGF-beta signaling negatively regulates neuronal morphogenesis through TGIF/Smad complex-mediated CRMP2 suppression. J Neurosci 38(20):4791–4810. https://doi.org/10.1523/JNEUROSCI.2423-17.2018
Xu P, Liu J, Derynck R (2012) Post-translational regulation of TGF-beta receptor and Smad signaling. FEBS Lett 586(14):1871–1884. https://doi.org/10.1016/j.febslet.2012.05.010
Xu P, Lin X, Feng XH (2016) Posttranslational regulation of Smads. Cold Spring Harb Perspect Biol 8(12). https://doi.org/10.1101/cshperspect.a022087
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK et al (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131(6):1164–1178. https://doi.org/10.1016/j.cell.2007.10.036
Stephan AH, Barres BA, Stevens B (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35:369–389. https://doi.org/10.1146/annurev-neuro-061010-113810
Yu W, Du Y, Zou Y, Wang X, Stephani U, Lu Y (2017) Smad anchor for receptor activation contributes to seizures in temporal lobe epilepsy. Synapse 71(3). https://doi.org/10.1002/syn.21957
Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z et al (2007) Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol 2007:76396. https://doi.org/10.1155/2007/76396
von Bernhardi R, Cornejo F, Parada GE, Eugenin J (2015) Role of TGFbeta signaling in the pathogenesis of Alzheimer’s disease. Front Cell Neurosci 9:426. https://doi.org/10.3389/fncel.2015.00426
Mogi M, Harada M, Kondo T, Narabayashi H, Riederer P, Nagatsu T (1995) Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson’s disease. Neurosci Lett 193(2):129–132
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12(10):585–601. https://doi.org/10.1038/nrn3085
Burke SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7(1):30–40. https://doi.org/10.1038/nrn1809
Coleman PD, Flood DG (1987) Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging 8(6):521–545
Yeoman M, Scutt G, Faragher R (2012) Insights into CNS ageing from animal models of senescence. Nat Rev Neurosci 13(6):435–445. https://doi.org/10.1038/nrn3230
Soreq L, Rose J, Soreq E, Hardy J, Trabzuni D, Cookson MR, Smith C, Ryten M et al (2017) Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep 18(2):557–570. https://doi.org/10.1016/j.celrep.2016.12.011
Mostany R, Anstey JE, Crump KL, Maco B, Knott G, Portera-Cailliau C (2013) Altered synaptic dynamics during normal brain aging. J Neurosci 33(9):4094–4104. https://doi.org/10.1523/JNEUROSCI.4825-12.2013
Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, Frost JL, Le KX et al (2015) Complement C3-deficient mice fail to display age-related hippocampal decline. J Neurosci 35(38):13029–13042. https://doi.org/10.1523/JNEUROSCI.1698-15.2015
Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20(17):6587–6593
Wallace M, Frankfurt M, Arellanos A, Inagaki T, Luine V (2007) Impaired recognition memory and decreased prefrontal cortex spine density in aged female rats. Ann N Y Acad Sci 1097:54–57. https://doi.org/10.1196/annals.1379.026
Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH (2010) Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci 30(22):7507–7515. https://doi.org/10.1523/JNEUROSCI.6410-09.2010
Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: friend or foe? Mol Neurobiol 54(10):8071–8089. https://doi.org/10.1007/s12035-016-0297-1
Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR (1998) Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging 19(5):497–503
Tremblay ME, Zettel ML, Ison JR, Allen PD, Majewska AK (2012) Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia 60(4):541–558. https://doi.org/10.1002/glia.22287
Robillard KN, Lee KM, Chiu KB, MacLean AG (2016) Glial cell morphological and density changes through the lifespan of rhesus macaques. Brain Behav Immun 55:60–69. https://doi.org/10.1016/j.bbi.2016.01.006
Rodriguez JJ, Yeh CY, Terzieva S, Olabarria M, Kulijewicz-Nawrot M, Verkhratsky A (2014) Complex and region-specific changes in astroglial markers in the aging brain. Neurobiol Aging 35(1):15–23. https://doi.org/10.1016/j.neurobiolaging.2013.07.002
Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, Klooster J, Bossers K et al (2014) Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging 35(1):1–14. https://doi.org/10.1016/j.neurobiolaging.2013.07.008
Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H (2011) Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34(1):3–11. https://doi.org/10.1111/j.1460-9568.2011.07738.x
Davies DS, Ma J, Jegathees T, Goldsbury C (2017) Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol 27(6):795–808. https://doi.org/10.1111/bpa.12456
Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K (2007) Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55(4):412–424. https://doi.org/10.1002/glia.20468
Norden DM, Fenn AM, Dugan A, Godbout JP (2014) TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia 62(6):881–895. https://doi.org/10.1002/glia.22647
Norden DM, Trojanowski PJ, Walker FR, Godbout JP (2016) Insensitivity of astrocytes to interleukin 10 signaling following peripheral immune challenge results in prolonged microglial activation in the aged brain. Neurobiol Aging 44:22–41. https://doi.org/10.1016/j.neurobiolaging.2016.04.014
Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS (2010) TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7(62):62. https://doi.org/10.1186/1742-2094-7-62
Tichauer JE, Flores B, Soler B, Eugenin-von Bernhardi L, Ramirez G, von Bernhardi R (2014) Age-dependent changes on TGFbeta1 Smad3 pathway modify the pattern of microglial cell activation. Brain Behav Immun 37:187–196. https://doi.org/10.1016/j.bbi.2013.12.018
Werry EL, Enjeti S, Halliday GM, Sachdev PS, Double KL (2010) Effect of age on proliferation-regulating factors in human adult neurogenic regions. J Neurochem 115(4):956–964. https://doi.org/10.1111/j.1471-4159.2010.06992.x
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2017) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol 54(4):2969–2985. https://doi.org/10.1007/s12035-016-9880-8
Hillen AEJ, Burbach JPH, Hol EM (2018) Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog Neurobiol 165-167:66–86. https://doi.org/10.1016/j.pneurobio.2018.02.002
Garrett AM, Weiner JA (2009) Control of CNS synapse development by {gamma}-protocadherin-mediated astrocyte-neuron contact. J Neurosci 29(38):11723–11731. https://doi.org/10.1523/JNEUROSCI.2818-09.2009
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. https://doi.org/10.1056/NEJMra0909142
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054):184–185
Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL (2007) Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci 27(4):796–807. https://doi.org/10.1523/JNEUROSCI.3501-06.2007
De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL (2007) Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem 282(15):11590–11601. https://doi.org/10.1074/jbc.M607483200
De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, Bigio EH, Jerecic J et al (2008) Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by a beta oligomers. Neurobiol Aging 29(9):1334–1347. https://doi.org/10.1016/j.neurobiolaging.2007.02.029
Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL et al (2002) Soluble oligomers of beta amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res 924(2):133–140
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95(11):6448–6453
Rodriguez JJ, Butt AM, Gardenal E, Parpura V, Verkhratsky A (2016) Complex and differential glial responses in Alzheimer’s disease and ageing. Curr Alzheimer Res 13(4):343–358
Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ (2010) Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia 58(7):831–838. https://doi.org/10.1002/glia.20967
Beauquis J, Pavia P, Pomilio C, Vinuesa A, Podlutskaya N, Galvan V, Saravia F (2013) Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer’s disease. Exp Neurol 239:28–37. https://doi.org/10.1016/j.expneurol.2012.09.009
Kulijewicz-Nawrot M, Verkhratsky A, Chvatal A, Sykova E, Rodriguez JJ (2012) Astrocytic cytoskeletal atrophy in the medial prefrontal cortex of a triple transgenic mouse model of Alzheimer’s disease. J Anat 221(3):252–262. https://doi.org/10.1111/j.1469-7580.2012.01536.x
Yeh CY, Vadhwana B, Verkhratsky A, Rodriguez JJ (2011) Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease. ASN Neuro 3(5):271–279. https://doi.org/10.1042/AN20110025
Zenaro E, Piacentino G, Constantin G (2017) The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis 107:41–56. https://doi.org/10.1016/j.nbd.2016.07.007
Merlini M, Meyer EP, Ulmann-Schuler A, Nitsch RM (2011) Vascular beta-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAbeta mice. Acta Neuropathol 122(3):293–311. https://doi.org/10.1007/s00401-011-0834-y
Park R, Kook SY, Park JC, Mook-Jung I (2014) Abeta1-42 reduces P-glycoprotein in the blood-brain barrier through RAGE-NF-kappaB signaling. Cell Death Dis 5:e1299. https://doi.org/10.1038/cddis.2014.258
Yang J, Lunde LK, Nuntagij P, Oguchi T, Camassa LM, Nilsson LN, Lannfelt L, Xu Y et al (2011) Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J Alzheimers Dis 27(4):711–722. https://doi.org/10.3233/JAD-2011-110725
Orre M, Kamphuis W, Osborn LM, Jansen AH, Kooijman L, Bossers K, Hol EM (2014) Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol Aging 35(12):2746–2760. https://doi.org/10.1016/j.neurobiolaging.2014.06.004
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Birch AM (2014) The contribution of astrocytes to Alzheimer’s disease. Biochem Soc Trans 42(5):1316–1320. https://doi.org/10.1042/BST20140171
Estrada LD, Oliveira-Cruz L, Cabrera D (2017) Transforming growth factor beta type I role in neurodegeneration: implications for Alzheimer s disease. Curr Protein Pept Sci 19:1180–1188. https://doi.org/10.2174/1389203719666171129094937
Chao CC, Hu S, Frey WH 2nd, Ala TA, Tourtellotte WW, Peterson PK (1994) Transforming growth factor beta in Alzheimer’s disease. Clin Diagn Lab Immunol 1(1):109–110
Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P (2002) Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol Aging 23(2):237–243
Mocali A, Cedrola S, Della Malva N, Bontempelli M, Mitidieri VA, Bavazzano A, Comolli R, Paoletti F et al (2004) Increased plasma levels of soluble CD40, together with the decrease of TGF beta 1, as possible differential markers of Alzheimer disease. Exp Gerontol 39(10):1555–1561. https://doi.org/10.1016/j.exger.2004.07.007
Flanders KC, Lippa CF, Smith TW, Pollen DA, Sporn MB (1995) Altered expression of transforming growth factor-beta in Alzheimer’s disease. Neurology 45(8):1561–1569
Chong JR, Chai YL, Lee JH, Howlett D, Attems J, Ballard CG, Aarsland D, Francis PT et al (2017) Increased transforming growth factor beta2 in the neocortex of Alzheimer’s disease and dementia with Lewy bodies is correlated with disease severity and soluble Abeta42 load. J Alzheimers Dis 56(1):157–166. https://doi.org/10.3233/JAD-160781
Hashimoto Y, Nawa M, Chiba T, Aiso S, Nishimoto I, Matsuoka M (2006) Transforming growth factor beta2 autocrinally mediates neuronal cell death induced by amyloid-beta. J Neurosci Res 83(6):1039–1047. https://doi.org/10.1002/jnr.20804
Harris-White ME, Balverde Z, Lim GP, Kim P, Miller SA, Hammer H, Galasko D, Frautschy SA (2004) Role of LRP in TGFbeta2-mediated neuronal uptake of Abeta and effects on memory. J Neurosci Res 77(2):217–228. https://doi.org/10.1002/jnr.20149
Tapella L, Cerruti M, Biocotino I, Stevano A, Rocchio F, Canonico PL, Grilli M, Genazzani AA et al (2018) TGF-beta2 and TGF-beta3 from cultured beta-amyloid-treated or 3xTg-AD-derived astrocytes may mediate astrocyte-neuron communication. Eur J Neurosci 47(3):211–221. https://doi.org/10.1111/ejn.13819
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q et al (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352(6286):712–716. https://doi.org/10.1126/science.aad8373
Afagh A, Cummings BJ, Cribbs DH, Cotman CW, Tenner AJ (1996) Localization and cell association of C1q in Alzheimer’s disease brain. Exp Neurol 138(1):22–32. https://doi.org/10.1006/exnr.1996.0043
Masuda T, Itoh J, Koide T, Tomidokoro Y, Takei Y, Ishii K, Tamaoka A (2017) Transforming growth factor-beta1 in the cerebrospinal fluid of patients with distinct neurodegenerative diseases. J Clin Neurosci 35:47–49. https://doi.org/10.1016/j.jocn.2016.09.018
Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L (1997) Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer’s disease. Nature 389(6651):603–606. https://doi.org/10.1038/39321
Lesne S, Docagne F, Gabriel C, Liot G, Lahiri DK, Buee L, Plawinski L, Delacourte A et al (2003) Transforming growth factor-beta 1 potentiates amyloid-beta generation in astrocytes and in transgenic mice. J Biol Chem 278(20):18408–18418. https://doi.org/10.1074/jbc.M300819200
Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E (2000) Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol 156(1):139–150
Grammas P, Ovase R (2002) Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer’s disease brain. Am J Pathol 160(5):1583–1587
Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C et al (2013) ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A 110(19):E1807–E1816. https://doi.org/10.1073/pnas.1220484110
Bien-Ly N, Gillespie AK, Walker D, Yoon SY, Huang Y (2012) Reducing human apolipoprotein E levels attenuates age-dependent Abeta accumulation in mutant human amyloid precursor protein transgenic mice. J Neurosci 32(14):4803–4811. https://doi.org/10.1523/JNEUROSCI.0033-12.2012
Zheng JY, Sun J, Ji CM, Shen L, Chen ZJ, Xie P, Sun YZ, Yu RT (2017) Selective deletion of apolipoprotein E in astrocytes ameliorates the spatial learning and memory deficits in Alzheimer’s disease (APP/PS1) mice by inhibiting TGF-beta/Smad2/STAT3 signaling. Neurobiol Aging 54:112–132. https://doi.org/10.1016/j.neurobiolaging.2017.03.002
Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L (2001) TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med 7(5):612–618. https://doi.org/10.1038/87945
Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA (2008) Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med 14(6):681–687. https://doi.org/10.1038/nm1781
Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K (2002) TGF-beta and the regulation of neuron survival and death. J Physiol Paris 96(1–2):25–30
Meyers EA, Kessler JA (2017) TGF-beta family signaling in neural and neuronal differentiation, development, and function. Cold Spring Harb Perspect Biol 9(8). https://doi.org/10.1101/cshperspect.a022244
Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L et al (2006) Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest 116(11):3060–3069. https://doi.org/10.1172/JCI27341
Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ (2002) Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res 70(3):462–473. https://doi.org/10.1002/jnr.10351
Katsel PL, Davis KL, Haroutunian V (2005) Large-scale microarray studies of gene expression in multiple regions of the brain in schizophrenia and Alzheimer’s disease. Int Rev Neurobiol 63:41–82. https://doi.org/10.1016/S0074-7742(05)63003-6
Lee HG, Ueda M, Zhu X, Perry G, Smith MA (2006) Ectopic expression of phospho-Smad2 in Alzheimer’s disease: uncoupling of the transforming growth factor-beta pathway? J Neurosci Res 84(8):1856–1861. https://doi.org/10.1002/jnr.21072
Ueberham U, Ueberham E, Gruschka H, Arendt T (2006) Altered subcellular location of phosphorylated Smads in Alzheimer’s disease. Eur J Neurosci 24(8):2327–2334. https://doi.org/10.1111/j.1460-9568.2006.05109.x
Baig S, van Helmond Z, Love S (2009) Tau hyperphosphorylation affects Smad 2/3 translocation. Neuroscience 163(2):561–570. https://doi.org/10.1016/j.neuroscience.2009.06.045
Kim ES, Kim RS, Ren RF, Hawver DB, Flanders KC (1998) Transforming growth factor-beta inhibits apoptosis induced by beta-amyloid peptide fragment 25-35 in cultured neuronal cells. Brain Res Mol Brain Res 62(2):122–130
Ren RF, Flanders KC (1996) Transforming growth factors-beta protect primary rat hippocampal neuronal cultures from degeneration induced by beta-amyloid peptide. Brain Res 732(1–2):16–24
Caraci F, Tascedda F, Merlo S, Benatti C, Spampinato SF, Munafo A, Leggio GM, Nicoletti F et al (2016) Fluoxetine prevents Abeta1-42-induced toxicity via a paracrine signaling mediated by transforming-growth-factor-beta1. Front Pharmacol 7:389. https://doi.org/10.3389/fphar.2016.00389
Sortino MA, Chisari M, Merlo S, Vancheri C, Caruso M, Nicoletti F, Canonico PL, Copani A (2004) Glia mediates the neuroprotective action of estradiol on beta-amyloid-induced neuronal death. Endocrinology 145(11):5080–5086. https://doi.org/10.1210/en.2004-0973
Caraci F, Battaglia G, Busceti C, Biagioni F, Mastroiacovo F, Bosco P, Drago F, Nicoletti F et al (2008) TGF-beta 1 protects against Abeta-neurotoxicity via the phosphatidylinositol-3-kinase pathway. Neurobiol Dis 30(2):234–242. https://doi.org/10.1016/j.nbd.2008.01.007
Chen JH, Ke KF, Lu JH, Qiu YH, Peng YP (2015) Protection of TGF-beta1 against neuroinflammation and neurodegeneration in Abeta1-42-induced Alzheimer’s disease model rats. PLoS One 10(2):e0116549. https://doi.org/10.1371/journal.pone.0116549
Shen WX, Chen JH, Lu JH, Peng YP, Qiu YH (2014) TGF-beta1 protection against Abeta1-42-induced neuroinflammation and neurodegeneration in rats. Int J Mol Sci 15(12):22092–22108. https://doi.org/10.3390/ijms151222092
Murphy-Ullrich JE, Poczatek M (2000) Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 11(1–2):59–69
Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C (2004) Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int 65(2):459–468. https://doi.org/10.1111/j.1523-1755.2004.00395.x
Rama Rao KV, Curtis KM, Johnstone JT, Norenberg MD (2013) Amyloid-beta inhibits thrombospondin 1 release from cultured astrocytes: effects on synaptic protein expression. J Neuropathol Exp Neurol 72(8):735–744. https://doi.org/10.1097/NEN.0b013e31829bd082
Son SM, Nam DW, Cha MY, Kim KH, Byun J, Ryu H, Mook-Jung I (2015) Thrombospondin-1 prevents amyloid beta-mediated synaptic pathology in Alzheimer’s disease. Neurobiol Aging 36(12):3214–3227. https://doi.org/10.1016/j.neurobiolaging.2015.09.005
Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE (1993) GFAP mRNA increases with age in rat and human brain. Neurobiol Aging 14(5):421–429
Colombo JA, Gayol S, Yanez A, Marco P (1997) Immunocytochemical and electron microscope observations on astroglial interlaminar processes in the primate neocortex. J Neurosci Res 48(4):352–357
Pitt J, Wilcox KC, Tortelli V, Diniz LP, Oliveira MS, Dobbins C, Yu XW, Nandamuri S et al (2017) Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Abeta oligomers. Mol Biol Cell 28(20):2623–2636. https://doi.org/10.1091/mbc.E17-06-0416
Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14(11):1225–1236
Phatnani H, Maniatis T (2015) Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol 7(6). https://doi.org/10.1101/cshperspect.a020628
Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA et al (2013) Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12(3):342–353. https://doi.org/10.1016/j.stem.2012.12.015
Garcia O, Torres M, Helguera P, Coskun P, Busciglio J (2010) A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down’s syndrome. PLoS One 5(12):e14200. https://doi.org/10.1371/journal.pone.0014200
Diniz LP, Tortelli V, Garcia MN, Araujo AP, Melo HM, Silva GS, Felice FG, Alves-Leon SV et al (2014) Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia 62(12):1917–1931. https://doi.org/10.1002/glia.22713
Matias I, Diniz LP, Buosi A, Neves G, Stipursky J, Gomes FCA (2017) Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-beta1. Front Aging Neurosci 9:184. https://doi.org/10.3389/fnagi.2017.00184
Chung YC, Kim SR, Jin BK (2010) Paroxetine prevents loss of nigrostriatal dopaminergic neurons by inhibiting brain inflammation and oxidative stress in an experimental model of Parkinson’s disease. J Immunol 185(2):1230–1237. https://doi.org/10.4049/jimmunol.1000208
Ledo JH, Azevedo EP, Clarke JR, Ribeiro FC, Figueiredo CP, Foguel D, De Felice FG, Ferreira ST (2013) Amyloid-beta oligomers link depressive-like behavior and cognitive deficits in mice. Mol Psychiatry 18(10):1053–1054. https://doi.org/10.1038/mp.2012.168
Caraci F, Leggio GM, Salomone S, Drago F (2017) New drugs in psychiatry: focus on new pharmacological targets. F1000Res 6:397. https://doi.org/10.12688/f1000research.10233.1
Orgeta V, Tabet N, Nilforooshan R, Howard R (2017) Efficacy of antidepressants for depression in Alzheimer’s disease: systematic review and meta-analysis. J Alzheimers Dis 58(3):725–733. https://doi.org/10.3233/JAD-161247
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Departamento de Ciência e Tecnologia do Ministério da Saúde (Decit), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). This manuscript was edited by the American Journal Experts (AJE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diniz, L.P., Matias, I., Siqueira, M. et al. Astrocytes and the TGF-β1 Pathway in the Healthy and Diseased Brain: a Double-Edged Sword. Mol Neurobiol 56, 4653–4679 (2019). https://doi.org/10.1007/s12035-018-1396-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1396-y