Abstract
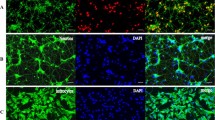
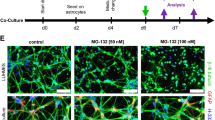
While astrocytes throughout the CNS share many common traits, they exhibit significant differences in function and number among brain regions. The aim of the present study is to assess the effect of region-specificity and number of astrocytes on the survival of dopaminergic neurons under stress, and to understand the possible mechanism by which these astrocytes extend neuroprotection to dopaminergic neurons. Purified astrocytes obtained from forebrain, midbrain, and hindbrain region were characterized through FACS and immunofluorescence. Co-culture experiments (using trans-wells) were then performed to measure the effect of region-specificities and numbers of astrocytes on primary midbrain culture under 6-OHDA stress. Cell survival augmented with an increase in astrocyte seeding number and total cell survival was comparable among the different region-specific astrocytes for all numbers. However, striking differences were observed in dopaminergic neuronal (TH) cell survival in the presence of midbrain astrocytes in comparison to forebrain and hindbrain astrocytes at all seeding numbers. At 75 μM 6-OHDA insult, while cell survival was comparable in purified astrocytes from the different brain regions, a distinct increase in BDNF secretion (significantly higher than its constitutive release) was noted for midbrain astrocytes compared to forebrain and hindbrain astrocytes. The TH immunopositive population decreased when TrkB inhibitor was added to the co-culture under 6-OHDA toxicity, suggesting that BDNF released by co-cultured astrocytes plays a key role in the survival of dopaminergic neurons. This BDNF release decreased in presence of NO inhibitor and increased in the presence of NO donor (DETA/NO). We conclude that the BDNF released from astrocytes under 6-OHDA toxicity is mediated through NO release through both autocrine and paracrine signaling, and this BDNF release is primarily responsible for the differential effect of region-specific astrocytes on TH neuron survival under these conditions.











Similar content being viewed by others
References
Liu B, Teschemacher AG, Kasparov S (2017) Neuroprotective potential of astroglia. J Neurosci Res. https://doi.org/10.1002/jnr.24140
Ota Y, Zanetti AT, Hallock RM (2013) The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast 2013:185463
Aschner M, Syversen T, Souza DO, Rocha JBT, Farina M (2007) Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res 40(3):285–291
Mehta B, Begum G, Joshi NB, Joshi PG (2008) Nitric oxide-mediated modulation of synaptic activity by astrocytic P2Y receptors. J Gen Physiol 132(3):339–349
Gupta S, Goswami P, Biswas J, Joshi N, Sharma S, Nath C, Singh S (2015) 6-Hydroxydopamine and lipopolysaccharides induced DNA damage in astrocytes: involvement of nitric oxide and mitochondria. Mutat Res Genet Toxicol Environ Mutagen 778:22–36
Roberts RA, Smith RA, Safe S, Szabo C, Tjalkens RB, Robertson FM (2010) Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology 276:85–94
Lian XY, Stringer JL (2004) Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res 1012(25):177–184
Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT et al (2012) Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337(6092):358–362
Garcia-Abreu J, Moura NV, Carvalho SL, Cavalcante LA (1995) Regionally specific properties of midbrain glia: interactions with midbrain neurons. J Neurosci Res 40:417–477
Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH (2012) Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26:891–907
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44
García-Marqués J, De Carlos JA, Greer CA, López-Mascaraque L (2010) 2010. Different astroglia permissivity controls the migration of olfactory bulb interneuron precursors. Glia 58(2):218–230
Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol 2:679–689
Mena MA, de Bernardo S, Casarejos MJ, Canals S, Rodriguez-Martin E (2002) The role of astroglia on the survival of dopamine neurons. Mol Neurobiol 25:245–263
Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C (2002) The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci 202(1–2):13–23
Anderson MA, Ao Y, Sofroniew MV (2014) Heterogeneity of reactive astrocytes. Neurosci Lett 565(17):23–29
Olude MA, Mustapha OA, Aderounmu OA, Olopade JO, Ihunwo AO (2015) Astrocyte morphology, heterogeneity, and density in the developing African giant rat (Cricetomys gambianus). Front Neuroanat 9:67
Savchenko VL, Nikonenko IR, Skibo GG, McKanna JA (1997) Distribution of microglia and astrocytes in different regions of the normal adult rat brain. Neurophysiology 29:343–351
Blomstrand F, Giaume C, Hansson E, Ronnback L (1999) Distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca2+ signaling. Am J Phys 277:C616–C627
Bordey A, Sontheimer H (2000) Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia 30(1):27–38
Saab AS et al (2012) Bergmann glial AMPA receptors are required for fine motor coordination. Science 337:749–753
Shinoda H, Marini AM, Cosi C, Schwartz JP (1989) Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science 245:415–417
Houades V, Koulakoff A, Ezan P, Seif I, Giaume C (2008) Gap junction-mediated astrocytic networks in the mouse barrel cortex. J Neurosci 28:5207–5217
Bernaudin M, Nouvelot A, MacKenzie ET, Petit E (1998) Selective neuronal vulnerability and specific glial reactions in hippocampal and neocortical organotypic cultures submitted to ischemia. Exp Neurol 150:30–39
Xu L, Sapolsky RM, Giffard RG (2001) Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp Neurol 169:416–424
Zhao G, Flavin MP (2000) Differential sensitivity of rat hippocampal and cortical astrocytes to oxygen-glucose deprivation injury. Neurosci Lett 285:177–180
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10(5):615–622
Van Damme P, Bogaert E, Dewil M, Hersmus N, Kiraly D, Scheveneels W, Bockx I, Braeken D et al (2007) Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc Natl Acad Sci U S A 104(37):14825–14830
Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, Cleveland DW, Goldstein LSB (2008) Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. PNAS 105:7594–7599
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(Pt 8):1437–1448
Fu Y, Paxinos G, Watson C, Halliday GM (2016) The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J Chem Neuroanat S0891-0618(16):30011–30014
Fearnley JM, Lees AJ (1991) Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114(Pt. 5):2283–2301
Rappold PM, Tieu K (2010) Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics 4:413–423
Vergun O, Keelan J, Khodorov BI, Duchen MR (1999) Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J Physiol 519(Pt 2):451–466
Schildge S, Bohrer C, Beck K, Schachtrup C (2013) Isolation and culture of mouse cortical astrocytes. J Vis Exp JoVE 71:50079
Ganapathy K, Sowmithra S, Bhonde R, Datta I (2016) By changing dimensionality, sequential culturing of midbrain cells, rather than two-dimensional culture, generates a neuron-glia ratio closer to in vivo adult midbrain. Cells Tissues Organs 201:445–463
Ganapathy K, Datta I, Sowmithra S, Joshi P, Bhonde R (2016) Influence of 6-hydroxydopamine toxicity on α-synuclein phosphorylation, resting vesicle expression, and vesicular dopamine release. J Cell Biochem 9999:1–18
Prasanna SJ, Saha B, Nandi D (2007) Involvement of oxidative and nitrosative stress in modulation of gene expression and functional responses by IFNγ. Int Immunol 19(7):867–879
Cheng A, Wang S, Cai J, Rao MS, Mattson MP (2003) Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 258:319–333
Hsieh H, Robertson CL, Vermehren-Schmaedick A, Balkowiec A (2010) Nitric oxide regulates BDNF release from nodose ganglion neurons in a pattern-dependent and cGMP-independent manner. J Neurosci Res 88(6):1285–1297
Double KL, Reyes S, Werry EL, Halliday GM (2010) Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog Neurobiol 92:316–329
Double KL (2012) Neuronal vulnerability in Parkinson’s disease. Parkinsonism Relat Disord 18:S52–S54
Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2:12
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127
Ouyang YB, Xu L, Liu S, Giffard RG (2014) Role of astrocytes in delayed neuronal death: GLT-1 and its novel regulation by microRNAs. Adv Neurobiol 11:171–188
Palmer TD, Willhoite AR, Gage FH (2000) Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494
Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM (1991) BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350:230–232
Feng L, Wang CY, Jiang H, Oho C, Mizuno K, Dugich-Djordjevic M, Lu B (1999) Differential effects of GDNF and BDNF on cultured ventral mesencephalic neurons. Brain Res Mol Brain Res 66:62–70
Baquet ZC, Bickford PC, Jones KR (2005) Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci 25(26):6251–6259
Baydyuk M, Xu B (2014) BDNF signaling and survival of striatal neurons. Front Cell Neurosci 8:254
Greenberg ME, Xu B, Lu B, Hempstead BL (2009) New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci: Off J Soc Neurosci 29(41):12764–12767
Bal-Price A, Moneer Z, Brown GC (2002) Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia 40:312–323
Ikeda H, Murase K (2004) Glial nitric oxide-mediated long-term presynaptic facilitation revealed by optical imaging in rat spinal dorsal horn. J Neurosci 24:9888–9896
Buskila Y, Amitai Y (2010) Astrocytic iNOS-dependent enhancement of synaptic release in mouse neocortex. J Neurophysiol 103:1322–1328
Xie AX, Petravicz J, McCarthy KD (2015) Molecular approaches for manipulating astrocytic signaling in vivo front. Cell Neurosci 9:144
Funding
This work is supported by the “Innovative Young Biotechnologist Award” achieved by I.D. from the Department of Biotechnology (DBT), Government of India, New Delhi; contract grant no. BT/07/IYBA/2013-7. K.G. is funded by Manipal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have declared that no competing interests exist.
Electronic Supplementary Material
Supplementary Fig. 1
Representative FACS histograms showing the percentage immunopositive β-tubulin III neuronal population, GFAP astrocyte population, and TH dopaminergic neuronal population in primary MB culture in control and 6-OHDA-treated conditions. (GIF 102 kb)
Supplementary Fig. 2
Representative dot plots of FACS analysis for annexin V/PI staining showing percentage of live cells, necrotic cells, early and late apoptotic cells in primary MB cells co-cultured with different numbers of astrocytes from three regions of the brain. (GIF 153 kb)
Supplementary Fig. 3
Characteristic FACS analysis of GFAP, β-tubulin III, and TH immunopositive population in primary MB culture under 6-OHDA stress and co-cultured with astrocytes (80,000) from three regions of the brain. (GIF 133 kb)
Supplementary Fig. 4
Representative FACS analysis of population immunopositive for VMAT2, α-synuclein (αSyn) and phosphorylated α-synuclein serine129 (pSyn) in primary MB cells co-cultured with 80,000 MB astrocytes under 6-OHDA stress with respect to primary MB culture in control and 6-OHDA-treated conditions. (GIF 148 kb)
Supplementary Fig. 5
Characteristic FACS histograms of the percentage immunopositive GFAP, TH, and β-tubulin III in primary MB cells co-cultured with midbrain astrocytes at the number of 80,000 under 6-OHDA stress with and without the TrkB inhibitor (ANA12) to demonstrate the effect of BDNF on the survival of dopaminergic neurons in primary MB culture. (GIF 185 kb)
Supplementary Fig. 6
Representative FACS histogram of population immunopositive for TH in primary midbrain cells co-cultured with 80,000 astrocytes from FB, MB, and HB. (GIF 138 kb)
Supplementary Fig. 7
Ethidium bromide-stained gel showing the expression of nNOS and iNOS in the FB, MB, and HB astrocytes under control and 6-OHDA stress. (GIF 43 kb)
Rights and permissions
About this article
Cite this article
Datta, I., Ganapathy, K., Razdan, R. et al. Location and Number of Astrocytes Determine Dopaminergic Neuron Survival and Function Under 6-OHDA Stress Mediated Through Differential BDNF Release. Mol Neurobiol 55, 5505–5525 (2018). https://doi.org/10.1007/s12035-017-0767-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0767-0