Abstract
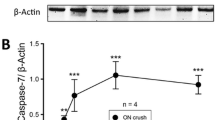
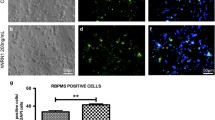
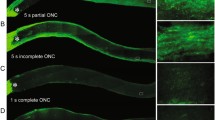
Axonal degeneration is one of the initial steps in many traumatic and neurodegenerative central nervous system (CNS) disorders and thus a promising therapeutic target. A focal axonal lesion is followed by acute axonal degeneration (AAD) of both adjacent axon parts, before proximal and distal parts follow different degenerative fates at later time points. Blocking calcium influx by calcium channel inhibitors was previously shown to attenuate AAD after optic nerve crush (ONC). However, it remains unclear whether the attenuation of AAD also promotes consecutive axonal regeneration. Here, we used a rat ONC model to study the effects of calcium channel inhibitors on axonal degeneration, retinal ganglion cell (RGC) survival, and axonal regeneration, as well as the molecular mechanisms involved. Application of calcium channel inhibitors attenuated AAD after ONC and preserved axonal integrity as visualized by live imaging of optic nerve axons. Consecutively, this resulted in improved survival of RGCs and improved axonal regeneration at 28 days after ONC. We show further that calcium channel inhibition attenuated lesion-induced calpain activation in the proximity of the crush and inhibited the activation of the c-Jun N-terminal kinase pathway. Pro-survival signaling via Akt in the retina was also increased. Our data thus show that attenuation of AAD improves consecutive neuronal survival and axonal regeneration and that calcium channel inhibitors could be valuable tools for therapeutic interventions in traumatic and degenerative CNS disorders.








Similar content being viewed by others
References
Lingor P, Koch JC, Tönges L, Bähr M (2012) Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res 349:289–311. doi:10.1007/s00441-012-1362-3
Coleman M (2005) Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci 6:889–898. doi:10.1038/nrn1788
Li H, Li S, Yu Z et al (2001) Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington’s disease mice. J Nuerosci 21:8473–8481
Fischer LR, Culver DG, Tennant P et al (2004) Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 185:232–240. doi:10.1016/j.expneurol.2003.10.004
Stokin GB, Lillo C, Falzone TL et al (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307:1282–1288. doi:10.1126/science.1105681
Burke RE, O’Malley K (2013) Axon degeneration in Parkinson’s disease. Exp Neurol 246:72–83. doi:10.1016/j.expneurol.2012.01.011
Medana IM, Esiri MM (2003) Axonal damage: a key predictor of outcome in human CNS diseases. Brain 126:515–530. doi:10.1093/brain/awg061
Yip PK, Malaspina A (2012) Spinal cord trauma and the molecular point of no return. Mol Neurodegener 7:6. doi:10.1186/1750-1326-7-6
Nickells RW, Howell GR, Soto I, John SWM (2012) Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci 35:153–179. doi:10.1146/annurev.neuro.051508.135728
Biousse V, Newman NJ (2001) Hereditary optic neuropathies. Ophthalmol Clin N Am 14:547–568. doi:10.1016/S0896-1549(05)70252-2
Costello F, Coupland S, Hodge W et al (2006) Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 59:963–969. doi:10.1002/ana.20851
Raff MC, Whitmore AV, Finn JT (2002) Axonal self-destruction and neurodegeneration. Science 296:868–871. doi:10.1126/science.1068613
Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T (2005) In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med 11:572–577. doi:10.1038/nm1229
Knöferle J, Koch JC, Ostendorf T et al (2010) Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A 107:6064–6069. doi:10.1073/pnas.0909794107
George EB, Glass JD, Griffin JW (1995) Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci 15:6445–6452. doi:10.1523/JNEUROSCI.5397-08.2009
Koch JC, Knöferle J, Tönges L, Ostendorf T, Bähr M, Lingor P (2010) Acute axonal degeneration in vivo is attenuated by inhibition of autophagy in a calcium-dependent manner. Autophagy 6(5):658–659. doi:10.4161/auto.6.5.12188
Ribas VT, Schnepf B, Challagundla M et al (2015) Early and sustained activation of autophagy in degenerating axons after spinal cord injury. Brain Pathol 25(2):157–170. doi:10.1111/bpa.12170
Koch JC, Knöferle J, Tönges L et al (2011) Imaging of rat optic nerve axons in vivo. Nat Protoc 6:1887–1896. doi:10.1038/nprot.2011.403
Ma M (2013) Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol Dis 60:61–79. doi:10.1016/j.nbd.2013.08.010
Roberts-Lewis JM, Savage MJ, Marcy VR et al (1994) Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J Neurosci 14:3934–3944
Ribas VT, Arruda-Carvalho M, Linden R, Chiarini LB (2011) Early c-Jun N-terminal kinase-dependent phosphorylation of activating transcription factor-2 is associated with degeneration of retinal ganglion cells. Neuroscience 180:64–74. doi:10.1016/j.neuroscience.2011.01.059
Fernandes KA, Harder JM, Fornarola LB et al (2012) JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death. Neurobiol Dis 46:393–401. doi:10.1016/j.nbd.2012.02.003
Yang J, Wu Z, Renier N et al (2015) Article pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell 160:161–176. doi:10.1016/j.cell.2014.11.053
Lingor P, Koeberle P, Kügler S, Bähr M (2005) Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain 128:550–558. doi:10.1093/brain/awh382
Pulverer BJ, Kyriakis JM, Avruch J et al (1991) Phosphorylation of c-jun mediated by MAP kinases. Nature 353:670–674. doi:10.1038/353670a0
Ahn J-Y (2014) Neuroprotection signaling of nuclear akt in neuronal cells. Exp Neurobiol 23:200–206
Christie KJ, Zochodne D (2013) Peripheral axon regrowth: new molecular approaches. Neuroscience 240:310–324. doi:10.1016/j.neuroscience.2013.02.059
Ertürk A, Hellal F, Enes J, Bradke F (2007) Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci Off J Soc Neurosci 27:9169–9180. doi:10.1523/JNEUROSCI.0612-07.2007
Beirowski B, Babetto E, Coleman MP, Martin KR (2008) The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci 28:1166–1179. doi:10.1111/j.1460-9568.2008.06426.x
Kitaoka Y, Munemasa Y, Kojima K et al (2013) Axonal protection by Nmnat3 overexpression with involvement of autophagy in optic nerve degeneration. Cell Death Dis 4, e860. doi:10.1038/cddis.2013.391
Yang P, Yang Z (2012) Enhancing intrinsic growth capacity promotes adult CNS regeneration. J Neurol Sci 312:1–6. doi:10.1016/j.jns.2011.08.037
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627. doi:10.1038/nrn1956
Pernet V, Joly S, Jordi N et al (2013) Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis 4:e734–11. doi:10.1038/cddis.2013.266
Williams PR, Marincu B, Sorbara CD et al (2014) A recoverable state of axon injury persists for hours after spinal cord contusion in vivo. Nat Commun 5:1–11. doi:10.1038/ncomms6683
Stirling DP, Cummins K, Wayne Chen SR, Stys P (2014) Axoplasmic reticulum Ca2+ release causes secondary degeneration of spinal axons. Ann Neurol 75:220–229. doi:10.1002/ana.24099
Villegas R, Martinez NW, Lillo J et al (2014) Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J Neurosci 34:7179–7189. doi:10.1523/JNEUROSCI.4784-13.2014
Henley J, Poo MM (2004) Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. doi:10.1016/j.tcb.2004.04.006
Hong K, Nishiyama M, Henley J (2000) Calcium signalling in the guidance of nerve growth by netrin-1. Nature 403:93–98
Kerstein PC, Jacques-fricke BT, Rengifo J et al (2013) Mechanosensitive TRPC1 channels promote calpain proteolysis of talin to regulate spinal axon outgrowth. J Neurosci 33:273–285. doi:10.1523/JNEUROSCI.2142-12.2013
Ghosh-roy A, Wu Z, Goncharov A et al (2010) Calcium and cyclic AMP promote axonal regeneration in caenorhabditis elegans and require DLK-1 Kinase. J Neurosci 30:3175–3183. doi:10.1523/JNEUROSCI.5464-09.2010
Fitzgerald M, Bartlett CA, Evill L et al (2009) Secondary degeneration of the optic nerve following partial transection: the benefits of lomerizine. Exp Neurol 216:219–230. doi:10.1016/j.expneurol.2008.11.026
Savigni DL, O’Hare Doig RL, Szymanski CR et al (2013) Three Ca2+ channel inhibitors in combination limit chronic secondary degeneration following neurotrauma. Neuropharmacology 75:380–390. doi:10.1016/j.neuropharm.2013.07.034
Lorber B, Tassoni A, Bull ND et al (2012) Retinal ganglion cell survival and axon regeneration in Wld S transgenic rats after optic nerve crush and lens injury. BMC Neurosci 6(13):56. doi:10.1186/1471-2202-13-56
Goll DE, Thompson VF, Li H et al (2003) The calpain system. Physiol Rev 83:731–801. doi:10.1152/physrev.00029.2002
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252. doi:10.1016/S0092-8674(00)00116-1
Mielke K, Herdegen T (2000) JNK and p38 stresskinases—degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol 61:45–60
Perrin V, Dufour N, Raoul C et al (2009) Implication of the JNK pathway in a rat model of Huntington’s disease. Exp Neurol 215:191–200. doi:10.1016/j.expneurol.2008.10.008
Saporito MS, Thomas BA, Scott RW (2000) MPTP activates c-Jun NH2-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J Neurochem 75:1200–1208. doi:10.1046/j.1471-4159.2000.0751200.x
Zhu X, Lee H, Raina AK et al (2002) The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals 11:270–281. doi:10.1159/000067426
Tezel G, Yang X, Yang J, Wax MB (2004) Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res 996:202–212. doi:10.1016/j.brainres.2003.10.029
Yoshida K, Behrens A, Le-Niculescu H et al (2002) Amino-terminal phosphorylation of c-Jun regulates apoptosis in the retinal ganglion cells by optic nerve transection. Investig Ophthalmol Vis Sci 43:1631–1635
Edström A, Ekström PAR (2003) Role of phosphatidylinositol 3-kinase in neuronal survival and axonal outgrowth of adult mouse dorsal root ganglia explants. J Neurosci Res 74:726–735. doi:10.1002/jnr.10686
Kügler S, Lingor P, Schöll U et al (2003) Differential transgene expression in brain cells in vivo and in vitro from AAV-2 vectors with small transcriptional control units. Virology 311:89–95. doi:10.1016/S0042-6822(03)00162-4
Michel U, Malik I, Ebert S et al (2005) Long-term in vivo and in vitro AAV-2-mediated RNA interference in rat retinal ganglion cells and cultured primary neurons. Biochem Biophys Res Commun 326:307–312. doi:10.1016/j.bbrc.2004.11.029
Acknowledgments
Paul Lingor was funded by the Else Kröner-Fresenius Foundation and the DFG-Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB). Vinicius Toledo Ribas is a fellow of the National Council for Scientific and Technological Development (CNPq), Brazil. We thank Elisabeth Barski and Vivian Dambeck for the excellent technical assistance. We thank Robert Siman from the University of Pennsylvania for the kind gift of the calpain-specific cleaved α-spectrin antibody.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were performed with the approval of the governmental authorities and according to the legislation of the local animal research council of the State of Lower Saxony (Braunschweig), Germany.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ribas, V.T., Koch, J.C., Michel, U. et al. Attenuation of Axonal Degeneration by Calcium Channel Inhibitors Improves Retinal Ganglion Cell Survival and Regeneration After Optic Nerve Crush. Mol Neurobiol 54, 72–86 (2017). https://doi.org/10.1007/s12035-015-9676-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9676-2